Novel n-acetylglucosamine-2-epimerase and method for producing cmp-neuraminic acid using the same
一种乙酰神经氨酸、乙酰葡糖胺的技术,应用在异构酶、生物化学设备和方法、酶等方向,能够解决N-乙酰甘露糖胺很贵、胞苷三磷酸产量低、纯化ManNAc过程复杂等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Example 1: Cloning and Transformation of Genes
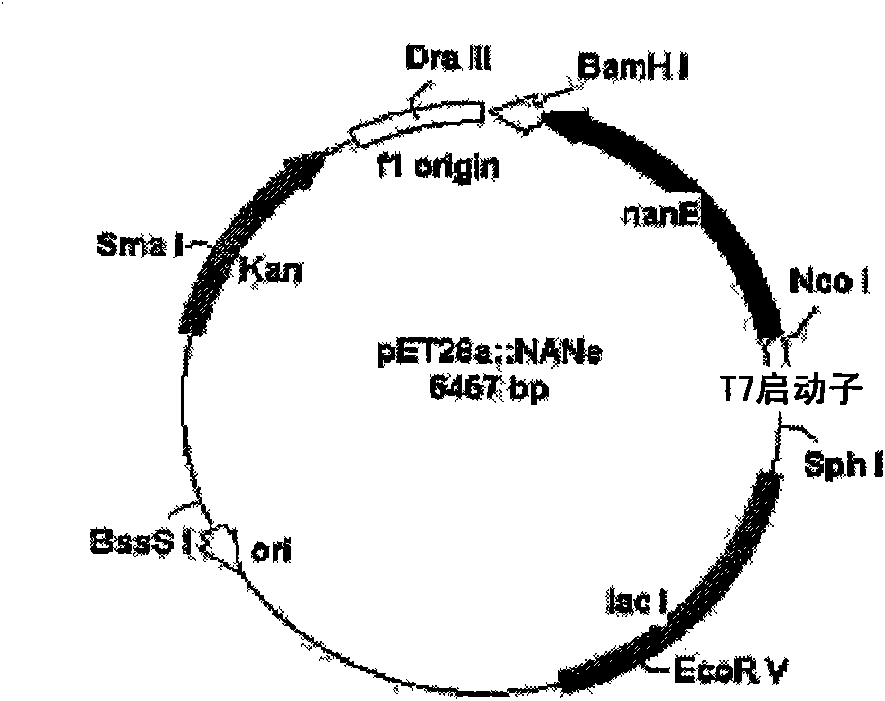
[0054] 1-1: nanE gene encoding N-acetylglucosamine-2-epimerase (SEQ ID NO: 1) cloning and transformation of
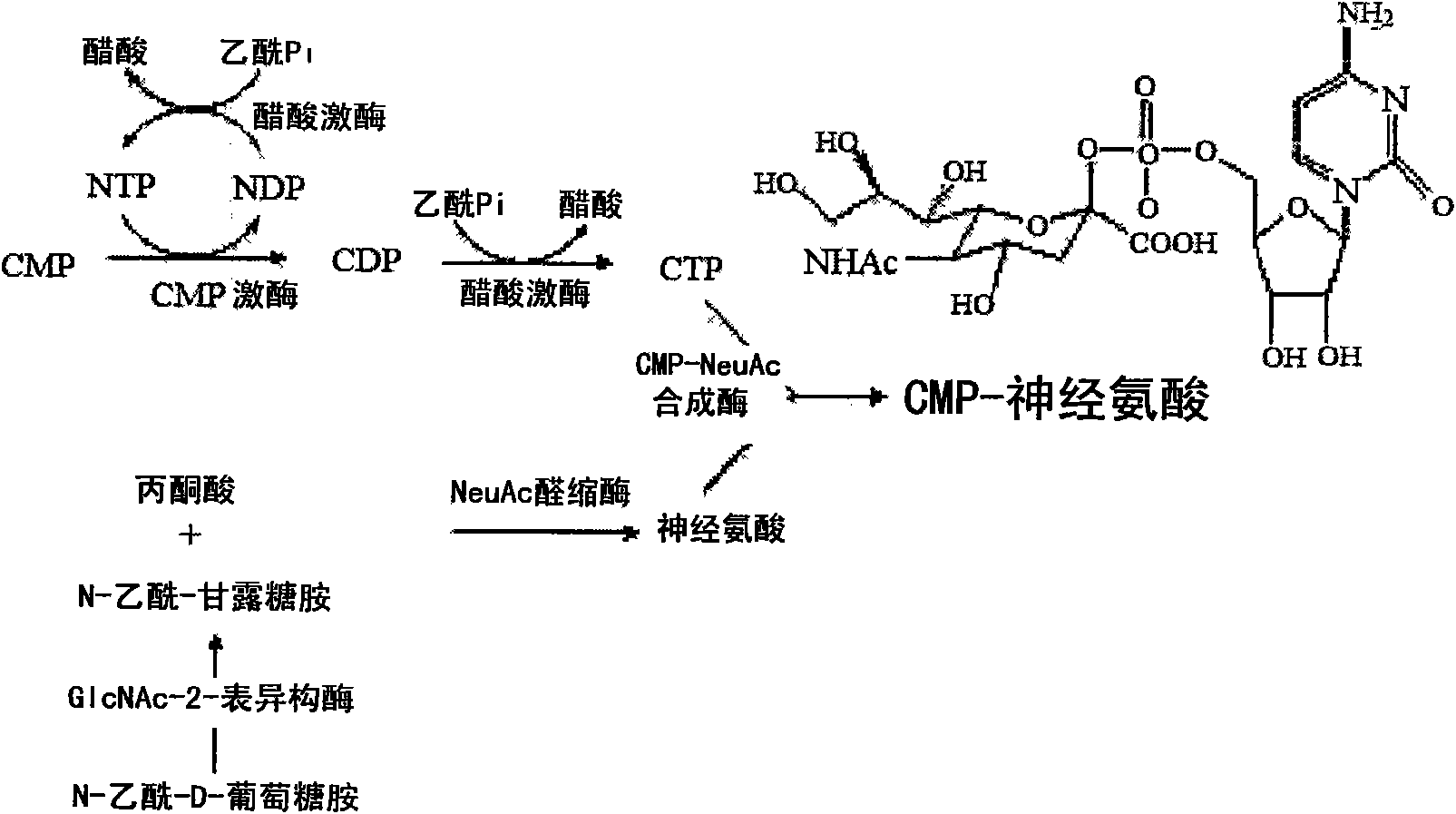
[0055] In order to eliminate the step of isomerizing N-acetyl-D-glucosamine into N-acetyl-D-mannosamine (which serves as the goal of increasing the production yield of CMP-N-acetylneuraminic acid and reducing production costs) Biggest problem pointed out), N-acetylglucosamine-2-epimerase (GlcNAc2 epimerase) was sought from various sources.
[0056] Three N-acetylglucosamine-2-epimerase-like genes were discovered from the anaerobic microorganism Bacteroides fragilis NCTC 9343 (NC_003228).
[0057] Candidate gene No.1 for N-acetylglucosamine-2-epimerase is located in the region of 947856-949034, has a size of 1179bp, and shows the same The corresponding genes of have 23.68-24.44% similarity.
[0058] Candidate gene No.2 for N-acetylglucosamine-2-epimerase is located in the scope of 1996625-1997809, has the ...
Embodiment 2
[0099] Example 2: Enzyme expression analysis
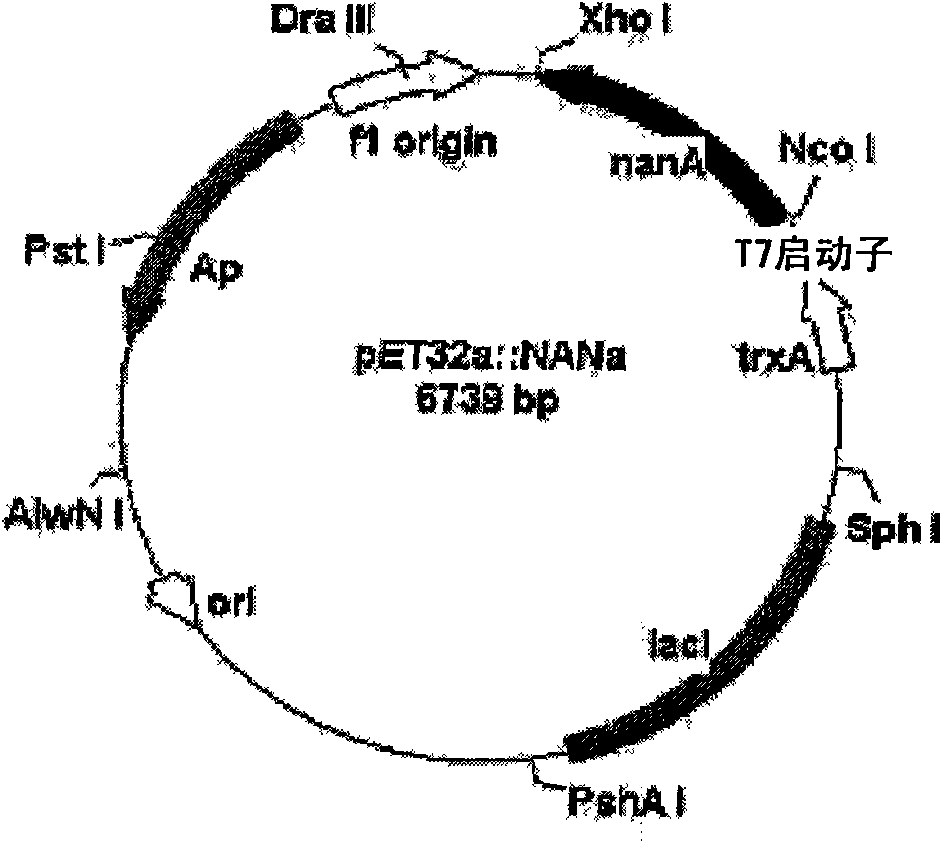
[0100] Inoculate 500 ml of seed culture obtained by culturing each transformant E.coli / pNANe, E.coli / pNANa, E.coli / pCMK, E.coli / pACKa and E.coli / pSYNb in LB medium to 5 liters of LB medium. When the cell concentration (OD 600 ) reached approximately 3-5, the cells were harvested. The culture conditions of the transformants containing various enzymes are shown in Table 1 below. Harvested cells were disrupted using a disruptor or French press, and the expression of each enzyme was analyzed by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) ( Figure 7 ).
[0101] As a result, it was observed that the enzyme encoded by the gene inserted into each transformant was expressed in a large amount.
[0102] Table 1: Culture conditions of strains producing each enzyme
[0103]
[0104] (CMK)
Embodiment 3
[0105] Embodiment 3: the purification of enzyme
[0106] The N-acetylglucosamine-2-epimerase (GlcAc2-isomerase), N-acetylneuraminic acid aldolase (NeuAc aldolase), cytidine 5'-phosphate kinase, acetate kinase and CMP-N-acetylneuraminic acid synthetase (CMP-NeuAc synthetase) were precipitated using ammonium sulfate and purified using an ion exchange resin column (Protein purification techniques "Protein purification techniques", 2nd edition, Oxford University Press, 2001).
[0107] The concentration of ammonium sulfate used for each enzyme precipitation can be properly adjusted in the range of 30-80%. Each enzyme is precipitated using ammonium sulfate in a refrigerator below 10°C, preferably 3-5°C, and stirred for 1-5 hours.
[0108] The precipitated enzyme was dissolved in a small amount of 50 mM Tris HCl (pH 7.5) buffer and desalted through a dialysis membrane. The purified and concentrated enzyme solution was applied on a UNO sphereQ (Bio-RAD) column packed with strong i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com