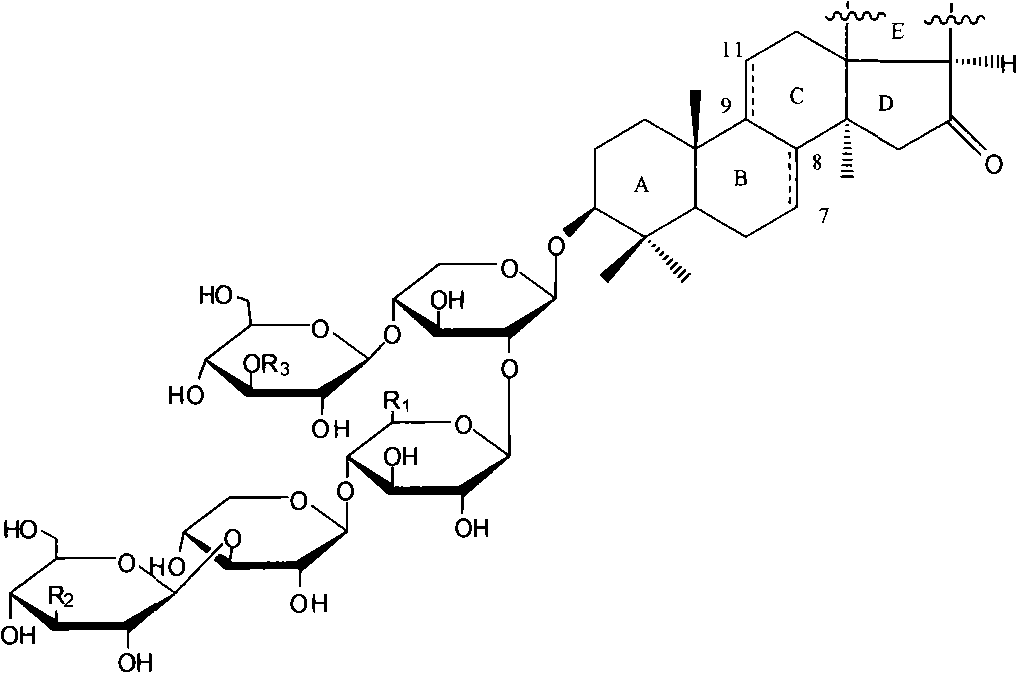
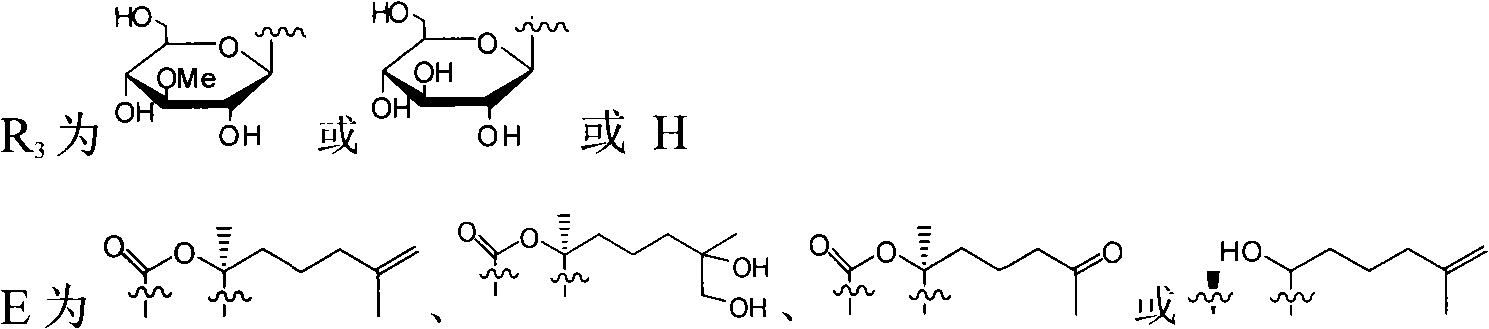
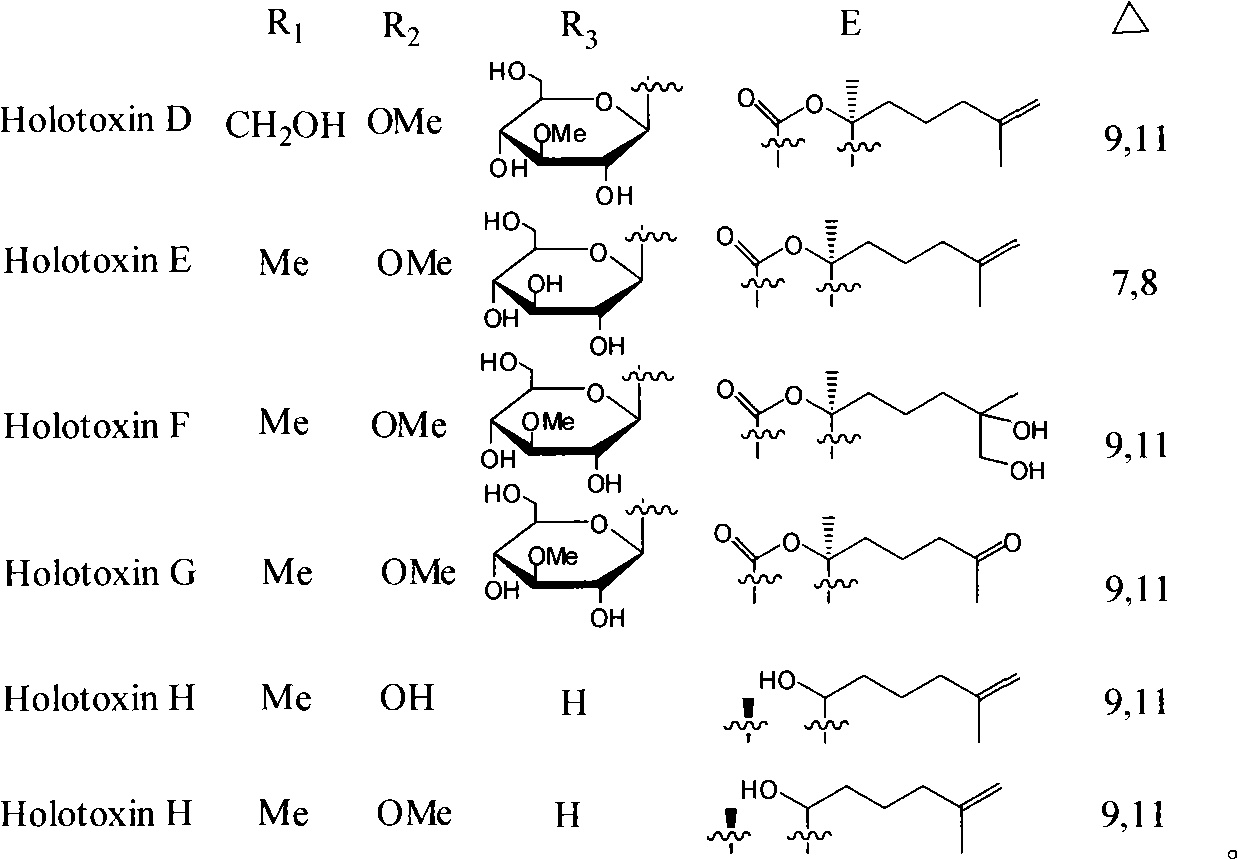
Triterpene glycosides antifungal compounds of sea cucumber HolotoxinD-I and preparation method thereof
A technology of triterpenoid saponins and compounds, applied in the field of medicine, can solve problems such as no antifungal activity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Example 1 Preparation of Holotoxin D~I from Apostichopus japonicus
[0047] The fresh freeze-dried imitation sea cucumber 3.0kg was pulverized into powder, and extracted with a 60% ethanol hot water bath with a weight ratio of 6 times. The extract was recovered under reduced pressure to obtain a liquid extract. The liquid extract was suspended in In water, pass through HP20 macroporous adsorption resin column, eluting with pure water, 70% ethanol and 95% ethanol respectively, collect 70% ethanol eluate, recover to no alcohol smell, and extract with 4 times the weight ratio of n-butanol Three times, the extracts were combined, the solvent was recovered and concentrated to dryness to obtain the total saponins extract of sea cucumber, which was then subjected to normal phase silica gel column chromatography and eluted with a solvent with a volume ratio of chloroform:methanol:water of 7:3:0.5 According to thin-layer chromatography detection, collect the saponins-containing fra...
Embodiment 2
[0048] Example 2 Preparation of Holotoxin D~I from Apostichopus japonicus
[0049] 4.5 kg of fresh freeze-dried imitation sea cucumber was pulverized into powder, and extracted with a 70% ethanol aqueous solution with a weight ratio of 5 times in a hot water bath. The extract was recovered under reduced pressure to obtain a liquid extract, and the liquid extract was suspended in In water, pass through DA101 macroporous adsorption resin column, eluting with pure water, 70% ethanol and 95% ethanol respectively, collect 70% ethanol eluate, recover to no alcohol smell, and extract with 4 times the weight ratio of n-butanol Three times, the extracts were combined, the solvent was recovered and concentrated to dryness to obtain the total saponins extract of sea cucumber, which was then subjected to normal phase silica gel column chromatography and eluted with a solvent with a volume ratio of chloroform: methanol: water of 6:4:0.8 According to thin-layer chromatography detection, collec...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com