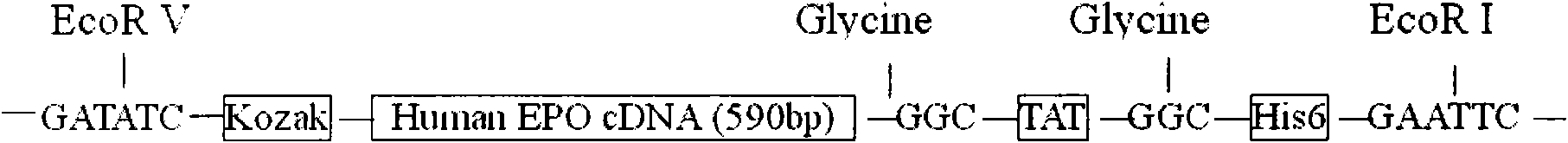Blood brain barrier penetrable erythropoietin (EPO) and application thereof
A technology of erythropoietin and blood-brain barrier, applied in erythropoietin, medical preparations containing active ingredients, cardiovascular system diseases, etc., can solve the problem of increased risk of large infarction, increased hematocrit, and increased micro-infarction To achieve the effect of prolonging the effective time window, reducing the volume of cerebral infarction, and strengthening neuroprotection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1: carrier preparation
[0028] 1. Amplification of human EPO cDNA
[0029] Human EPO cDNA encodes 193 amino acids. After the EPO primary protein is synthesized, the signal peptide containing 27 amino acid residues at the amino terminal becomes mature EPO after cleavage. Mature human EPO is thus 166 amino acids. Recent studies have found that the 166th amino acid arginine is also cleaved. Based on the above research results, when we constructed the fusion protein containing the HIV TAT transduction sequence, we added the HIV TAT and the histidine tag used for isolation and purification to the 3' end of the EPO cDNA, and at the same time added the 166th arginine delete. Human EPO cDNA containing the HIVTAT tag was amplified by PCR from a human kidney Marathon-ready cDNA library (Clontech, USA). Primers were designed according to the published human EPO cDNA sequence (gene sequence number: NM000799). The upstream sequence contains EcoRV and KOZAK sequence ...
Embodiment 2
[0032] Embodiment 2: for the establishment of the Chinese hamster ovary (CHO) stable cell line expressing EPO-TAT fusion protein and the preparation and purification of EPO-TAT fusion protein thereof
[0033] 1. Establishment of CHO stable cell lines expressing EPO-TAT fusion protein
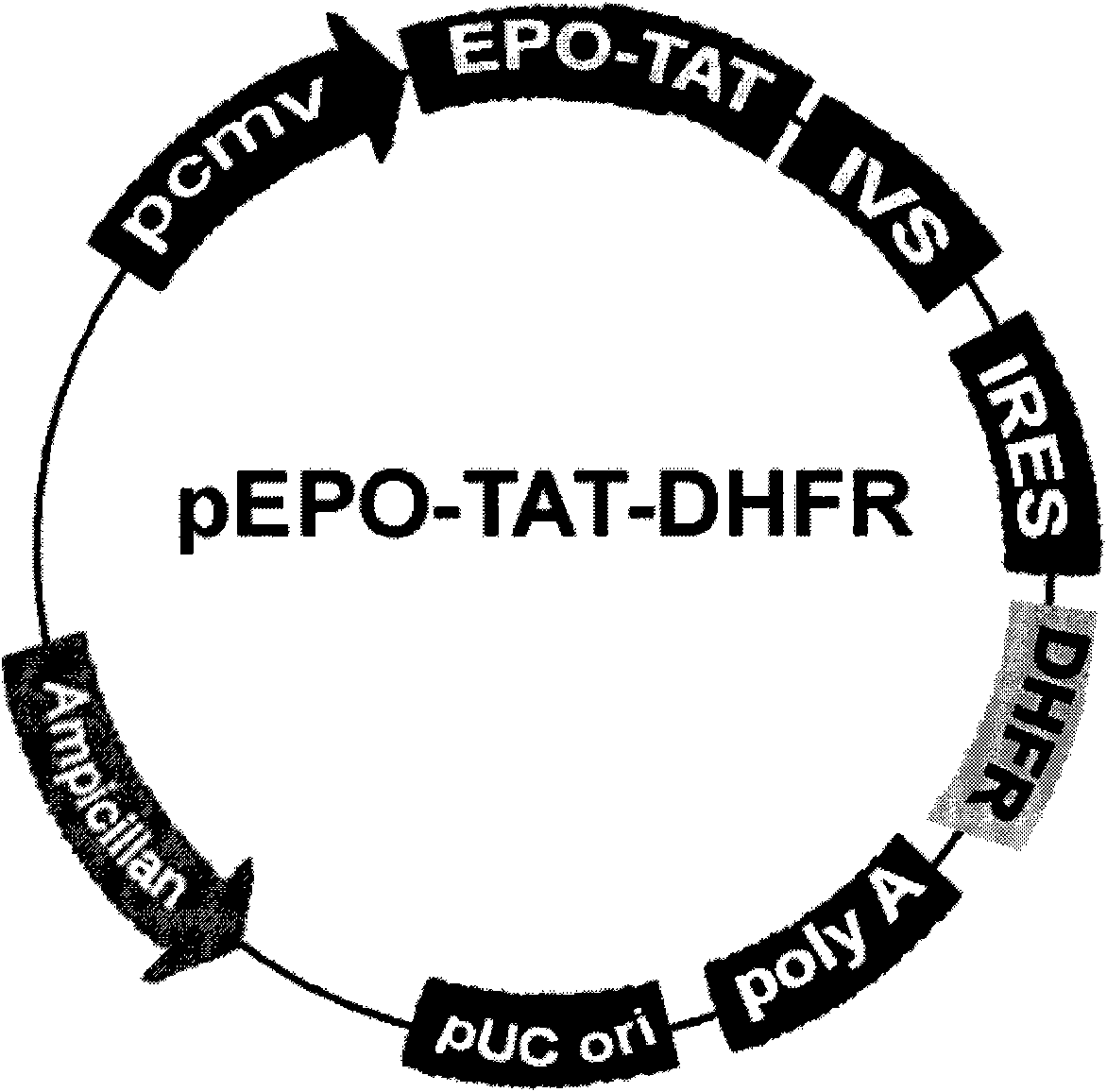
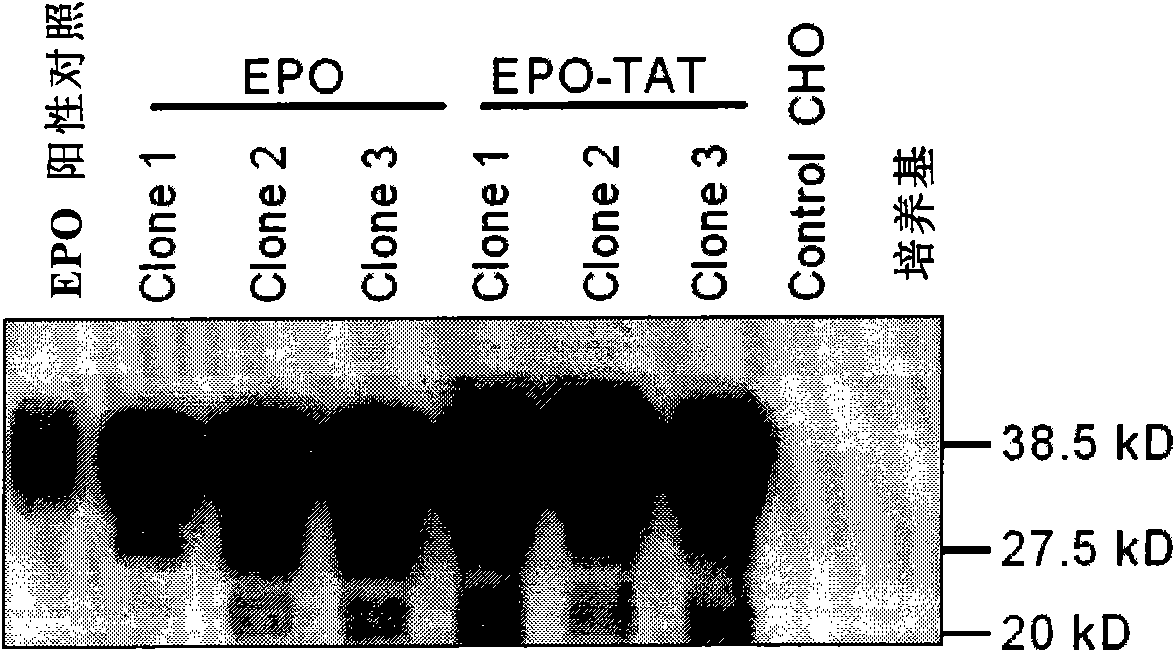
[0034] After the vector pEPO-TAT-DHFR was linearized with XhoI enzyme, it was recovered and purified. Using the transfection reagent Lipofectamine 2000 (Invitrogen, USA), the linearized plasmid was introduced into the DHFR-deleted CHO cell line DG44 (gifted by Duke University). After 24 hours of transfection, subculture at 1:12, change to nucleotide-free α-MEM medium (Invitrogen, USA) for culture, and add 5pM methotrene at the same time, select a single clone and transfer it to a 6-well plate after 2 weeks to continue to cultivate. Take 10 μl of culture medium and use Western blot or ELISA to identify EPO-TAT high-expressing cell lines after diluting 1000 times. Take six of the EPO-TAT high-e...
Embodiment 3
[0037] Example 3: Detect whether TAT-mediated EPO can increase EPO passing through the blood-brain barrier
[0038] The purpose of our preparation of TAT-labeled EPO is to improve its penetration of the blood-brain barrier and increase the therapeutic concentration of EPO in the brain. To further prove this problem, we intraperitoneally injected EPO-TAT or wild-type EPO (unfused TAT) to adult rats at a dose of 5000U / kg body weight. After 3 hours of injection, the plasma and cerebrospinal fluid were collected and the EPO content was determined using an ELISA kit (R&D System), the sensitivity of which was 0.8mU / ml. We found no difference in the sensitivity of this kit for measuring EPO-TAT and wild-type EPO. As shown in Figure 3, the content of EPO-TAT in blood was significantly higher than that of wild-type EPO. The monitoring results of cerebrospinal fluid samples showed that the efficiency of EPO-TAT passing through the BBB was significantly higher than that of wild-type EP...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com