(meth)acrylate compound, resin composition containing the same, cured product of the resin composition, and energy ray-curable resin composition for optical lens sheet and cured product thereof
A technology of resin composition and acrylate, which is applied in optics, optical components, instruments, etc., can solve the problems of high Tg point, poor substrate adhesion and mold release, and limited substrate types, etc., and achieve excellent mold release, good Effects of light resistance and excellent transparency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0135] Hereinafter, the present invention will be described in more detail through examples. It should be noted that the present invention is not limited by the following examples.
Synthetic example 1
[0136] The synthesis of synthetic example 1 epoxy resin (a-1)
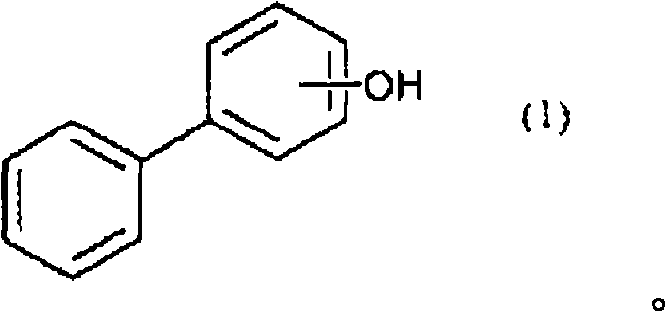
[0137] The flask equipped with a thermometer, a cooling tube, and a stirrer was purged with nitrogen, and 170 g of a compound of the following formula (2) (manufactured by O-PP Sanko Co., Ltd.), 370 g of epichlorohydrin, and 74 g of methanol were charged and dissolved. Furthermore, it heated to 70 degreeC, added 41 g of flaky sodium hydroxide in batches over 90 minutes, and reacted at 70 degreeC for 60 minutes after that. After the reaction is finished, wash twice with 200g of water to remove the generated salts, etc., and heat and reduce pressure (~70°C, -0.08MPa~-0.09MPa) and stir for 3 hours to remove the excess epichlorohydrin. And so on to remove by distillation. 450 g of methyl isobutyl ketone was added and dissolved in the residue, and the temperature was raised to 70°C. Add 10 g of a 10% by weight aqueous sodium hydroxide solution with stirring, react for 1 hour, wash with water until the washing water b...
Synthetic example 2
[0139] Synthesis of Synthesis Example 2 Epoxy Resin (a-2)
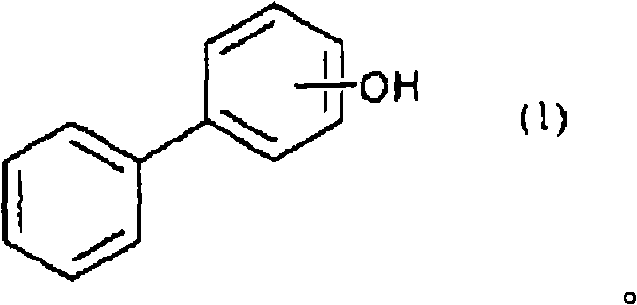
[0140] The flask equipped with a thermometer, a cooling tube, and a stirrer was purged with nitrogen, and 181 g of a compound of the following formula (3) (manufactured by P-PP Sanko Co., Ltd.), 394 g of epichlorohydrin, and 80 g of methanol were added and dissolved. Furthermore, it heated to 70 degreeC, added 44 g of flaky sodium hydroxide in batches over 90 minutes, and reacted at 70 degreeC for 60 minutes after that. After the reaction is finished, wash twice with 200g of water to remove the generated salts, etc., and heat and reduce pressure (~70°C, -0.08MPa~-0.09MPa) and stir for 3 hours to remove the excess epichlorohydrin. And so on to remove by distillation. 480 g of methyl isobutyl ketone was added and dissolved in the residue, and the temperature was raised to 70°C. 12 g of a 10% by weight aqueous sodium hydroxide solution was added with stirring, and after reacting for 1 hour, water was washed until the w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
| epoxy equivalent | aaaaa | aaaaa |
| epoxy equivalent | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com