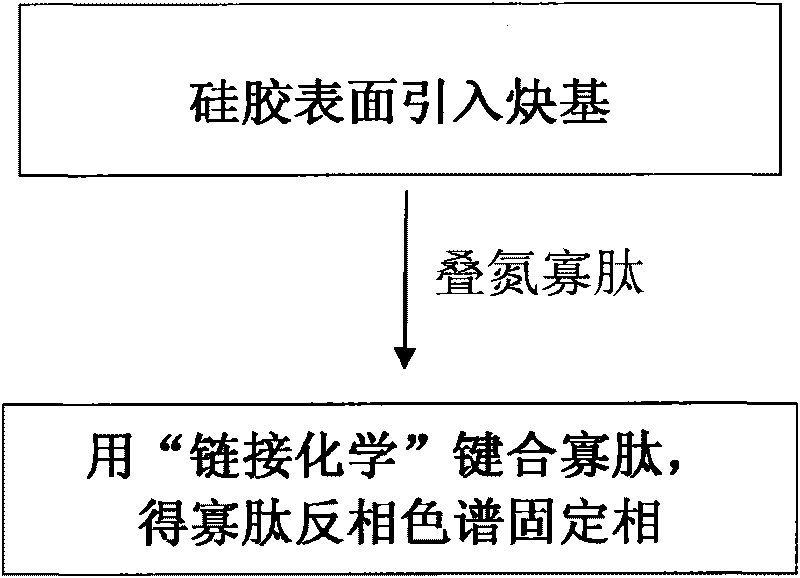
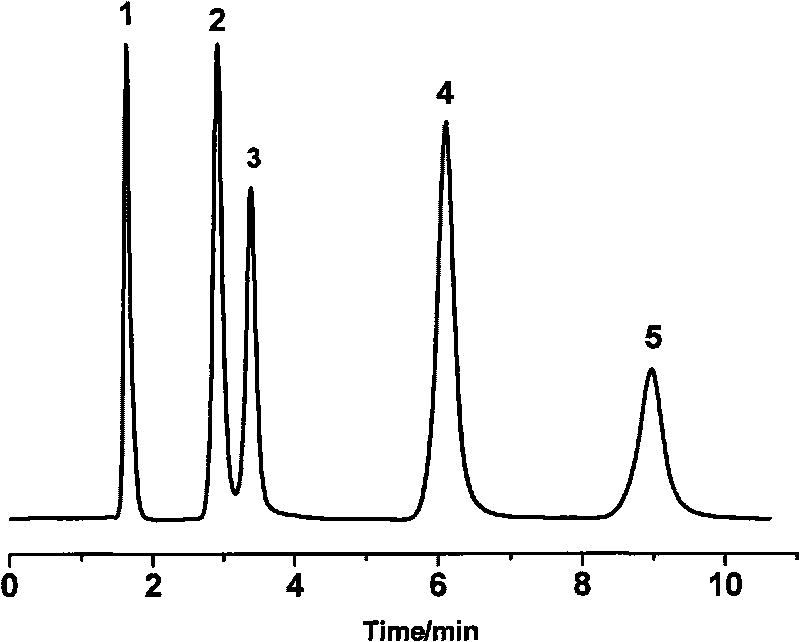
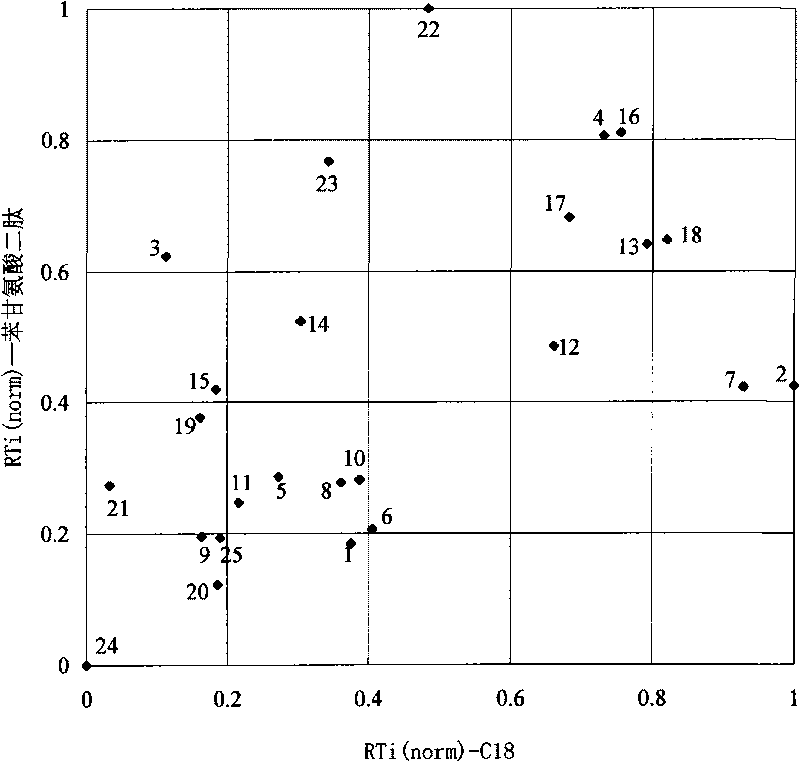
Oligopeptide phase reversal chromatography stationary phase and preparation method thereof
A technology of reversed-phase chromatography and stationary phase, applied in chemical instruments and methods, and other chemical processes, can solve problems such as scarcity, and achieve the effect of single reaction site, high selectivity, high conversion rate, and regular and orderly structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0040] An embodiment comprises the following steps:
[0041] (1) Preparation of Azidesulfonylimidazole Hydrochloride
[0042] Add 50mmol sulfonyl chloride (about 4mL) dropwise to 50mmol NaN in an ice-water bath 3 (about 3.2 g) in 100 mL of anhydrous MeCN suspension, the mixture was stirred overnight at room temperature;
[0043] Slowly add 100mmol imidazole (about 6.8g) into the above system under an ice-water bath, and stir the obtained emulsion at room temperature overnight;
[0044] Add 75mL of ethyl acetate to the mixture to dilute, successively wash with 2×75mL H 2 O, 2 x 120mL saturated NaHCO 3 The solution was extracted, and the organic phase was added with anhydrous Na 2 SO 4 Drying, suction filtration, and salt formation: Add HCl in EtOH solution (5.4mL acetyl chloride was added dropwise to 19mL EtOH under ice-water bath) to the above organic phase, a white solid was precipitated, suction filtration, washing with ethyl acetate, Obtain solid azidesulfonylimidazol...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com