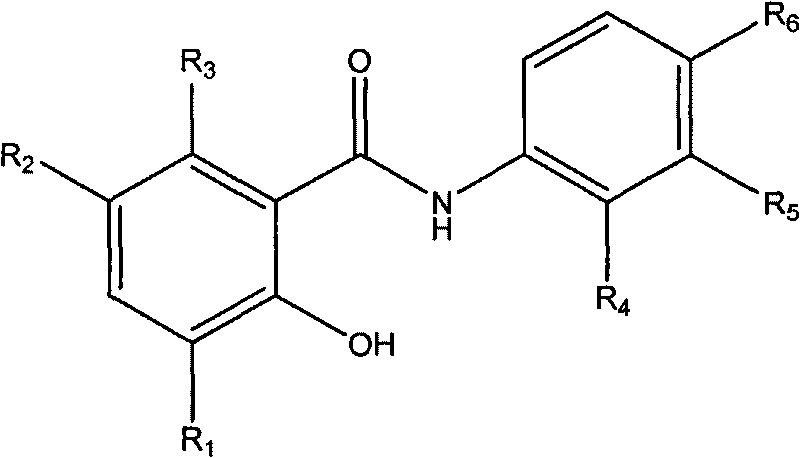
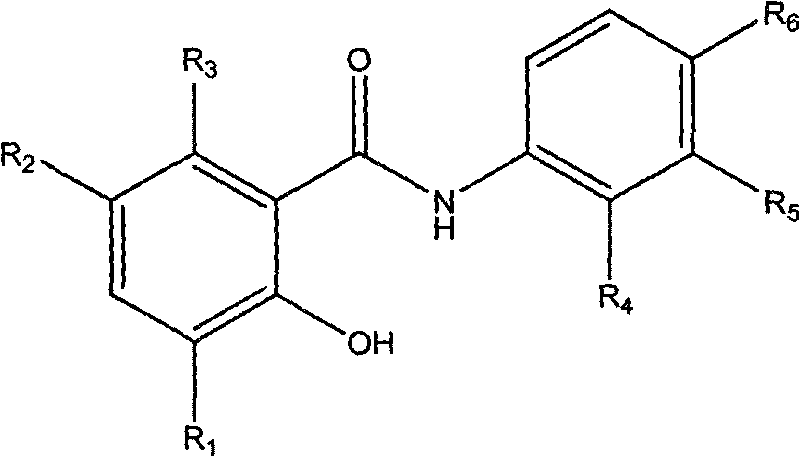
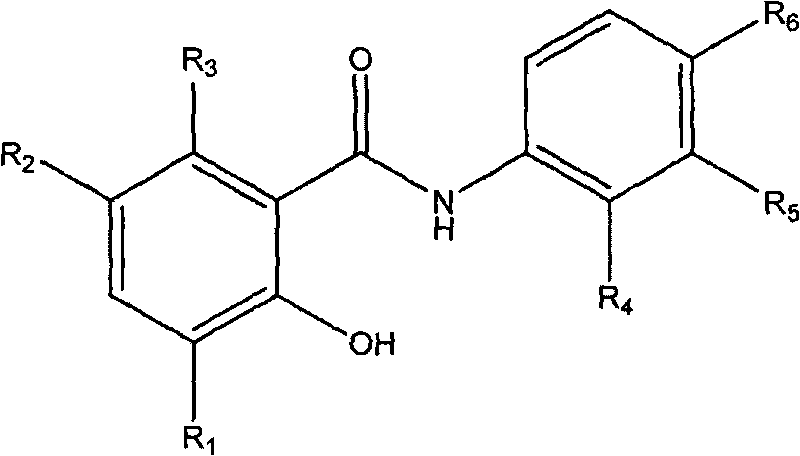
Salicylanilide derivatives, preparation method thereof and application thereof
A technology of salicylanilide and its derivatives, which is applied in the field of salicylanilide derivatives and their production methods and uses, can solve the problems of limited application, achieve strong specific binding ability, inhibit signal transduction and growth and division Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Example 1: Preparation of 2-hydroxy-6-methyl-N-phenylbenzamide (compound 1)
[0021]
[0022] Add 1mmol 6-methylsalicylic acid and 1mmol aniline, 100mg of 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC·HCl) in a 50ml single-necked round bottom flask as a catalyst, and add 5 ml of anhydrous CH 2 Cl 2 as a reaction solvent. Stir with a magnetic stirrer and heat to reflux at 40°C in an oil bath. After reacting for 8 hours, a clear reaction solution is obtained. Add 40ml of 200-300 mesh column chromatography silica gel to the reaction solution, and rotate under reduced pressure with a rotary evaporator to remove excess solvent and make it fully mixed. The target product was separated by column chromatography, and eluted with a mixed solvent of ethyl acetate:petroleum ether=1:3. The obtained eluent was again distilled under reduced pressure with a rotary evaporator to remove the solvent to obtain the target compound in the form of white powder. Yiel...
Embodiment 2
[0023] Example 2: Preparation of N-(2-bromophenyl)-2-hydroxyl-6-methylbenzamide (compound 2)
[0024]
[0025] The preparation method is the same as in Example 1. 2-Br aniline was used instead of aniline to give the target compound as a white powder. Yield 83%. Mp165-167℃. 1 H NMR (500MHz, d 6 -DMSO): 2.31 (s, 3H); 6.81-6.84 (m, 2H); 7.10-7.13 (m, 1H); 7.40-7.43 (m, 1H); 7.70 (dd, J 1 = 1.5Hz,J 2 =8.3Hz, 1H); 7.93(dd, J 1 = 1.5Hz,J 2 = 8.0Hz, 1H); 8.29(dd, J 1 = 1.5Hz,J 2 =8.3Hz, 1H); 10.68(s, 1H); 11.83(s, 1H).MS(ESI): 307.2(C 14 h 13 BrNO 2 ,[M+H] + ).Anal.Calcd for C 14 h 12 BrNO 2 : C, 54.92; H, 3.95; Br, 26.10; N, 4.58%; Found: C, 55.01; H, 3.94; Br, 26.07; N, 4.59%.
Embodiment 3
[0026] Example 3: Preparation of N-(4-fluorophenyl)-2-hydroxyl-6-methylbenzamide (compound 3)
[0027]
[0028]The preparation method is the same as in Example 1. 4-Fluoroaniline was substituted for aniline to give the target compound as a white powder. Yield 81%. Mp 135-137°C. 1 H NMR (300MHz, d 6 -DMSO): 2.30(s, 3H); 6.79(d, J=4.4Hz, 2H); 7.21(t, J=9.0Hz, 2H); 7.69-7.73(m, 2H); 7.89(d, J= 8.2Hz, 1H); 10.34(s, 1H); 11.90(s, 1H). MS(ESI): 246.3(C 14 h 13 FNO 2 ,[M+H] + ).Anal.Calcd for C 14 h 12 FNO 2 : C, 68.56; H, 4.93; F, 7.75; N, 5.71%; Found: C, 68.69; H, 4.89; F, 7.72; N, 5.69%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com