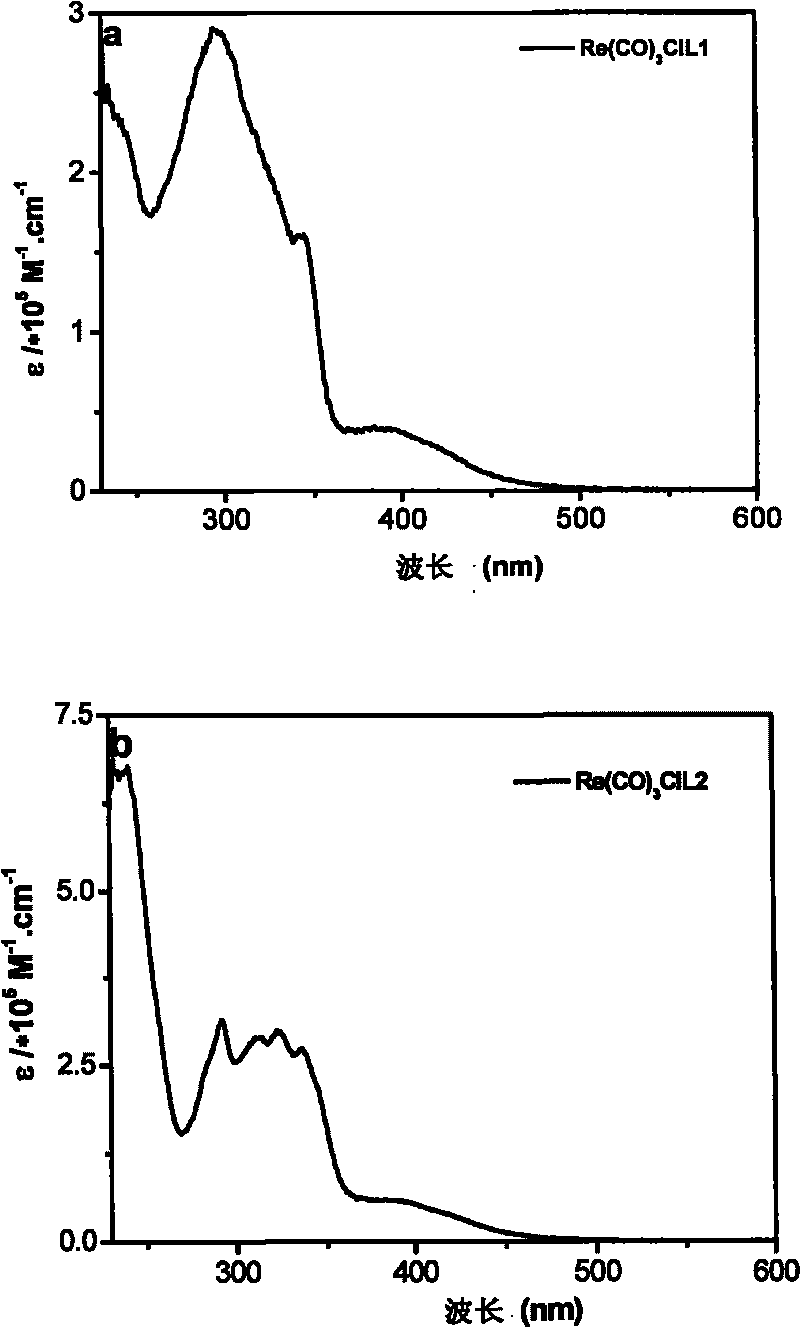
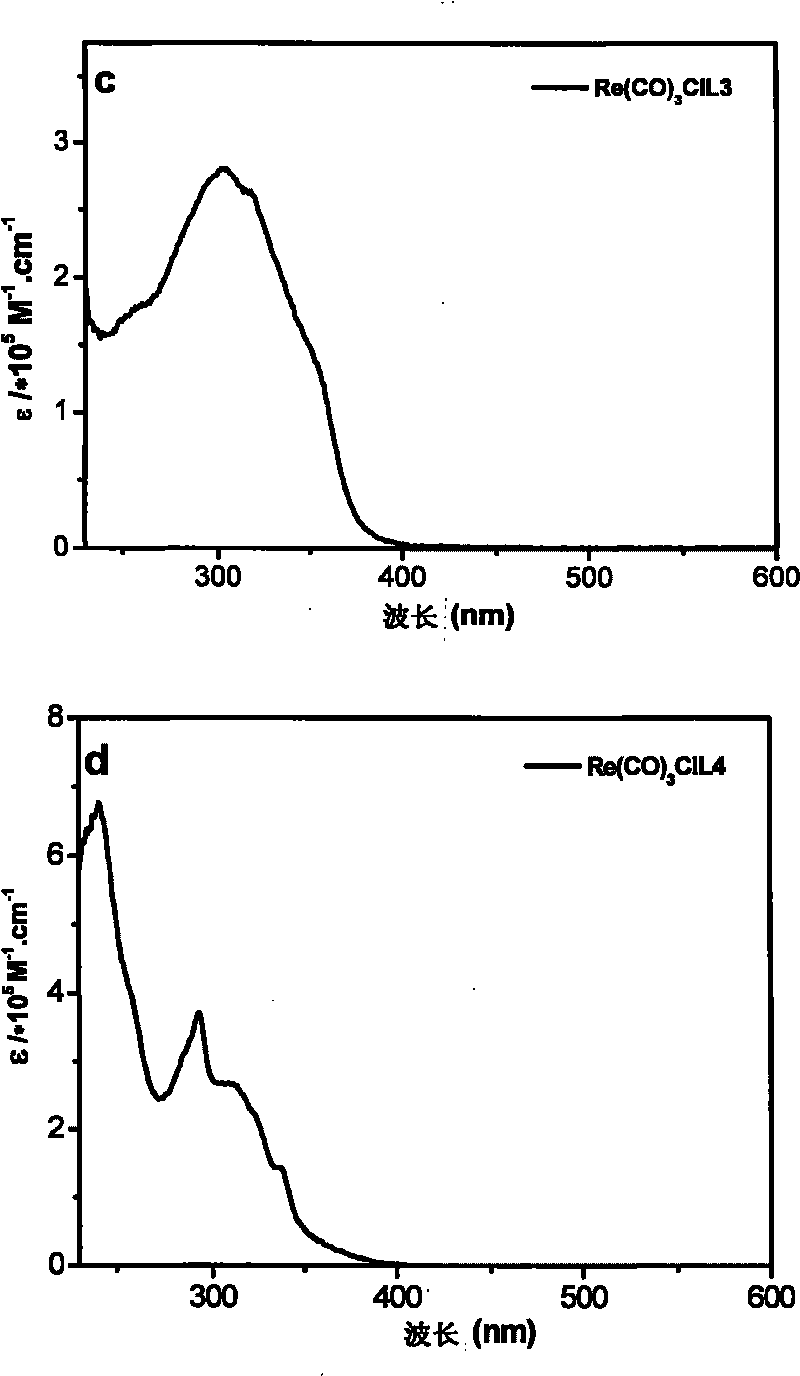
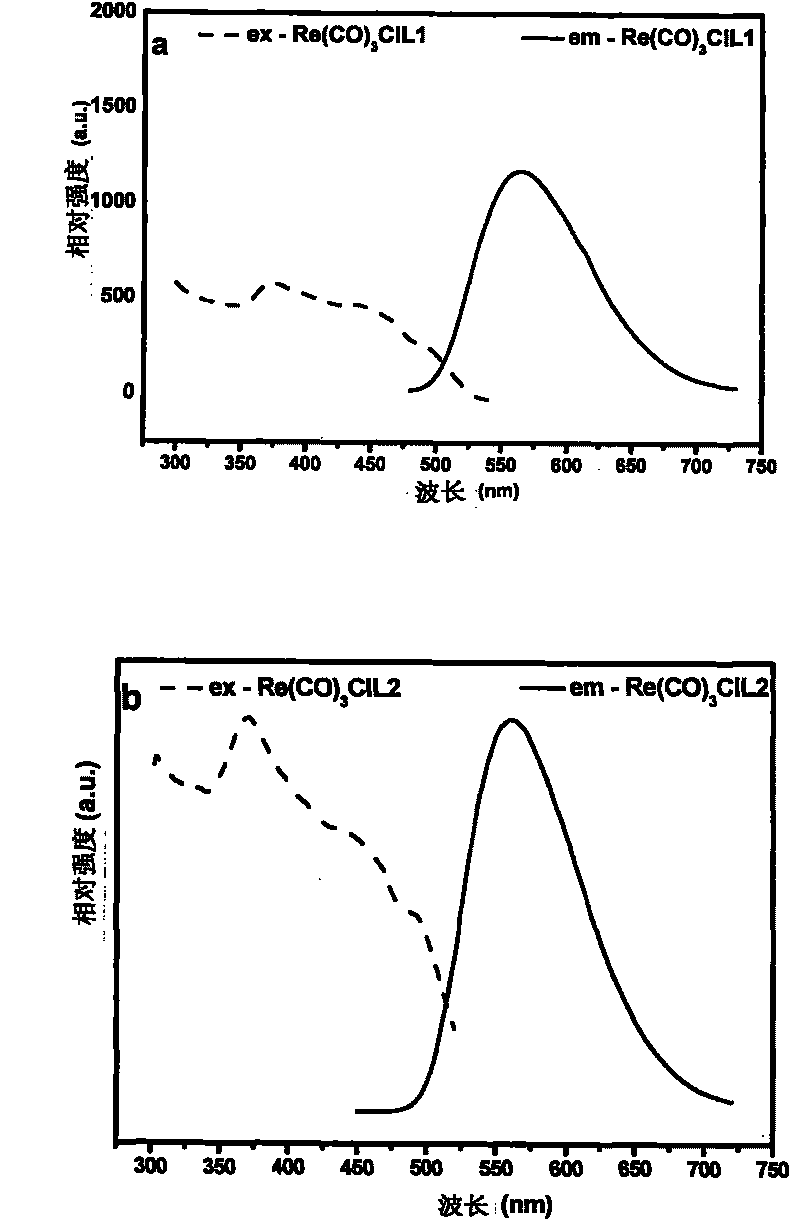
Tricarbonal rhenium (I) complexes containing carrier-transporting groups (oxadiazole or carbazole), preparation method and application thereof
A technology of oxadiazole phenyl and complexes, which is applied in the field of organic electroluminescent devices, can solve the problems of unreached carrier transport performance, annihilation, and long excited state lifetime, and achieve high yield, stable properties, and favorable The effect of purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1: Tricarbonyl [1-(4-5'-phenyl-1,3,4-oxadiazolyl)-2-(2-pyridyl)benzimidazole] rhenium(I) chloride , tricarbonyl chloride [1-(4-N-carbazolylphenyl)-2-(2-pyridyl) benzimidazole] rhenium (I), tricarbonyl chloride [N-(4-5' -Phenyl-1,3,4-oxadiazolephenyl)-2,2'-dipyridylamine] rhenium (I) and tricarbonyl chloride [N-(4-N-carbazolylphenyl) Preparation of -2,2'-dipyridylamine]rhenium(I)
[0029] 1. Preparation of 2-(4-bromophenyl)-5-phenyl-1,3,4-oxadiazole [2-(4-bromophenyl)-5-phenyl-1,3,4-oxadiazole Azole is commercially available, CAS number: 21510-43-0].
[0030] At room temperature, 0.1 mole of 4-bromobenzoyl chloride was added dropwise to a solution of 0.1 mole of benzohydrazide and 0.1 mole of triethylamine in 150 ml of chloroform, stirred for 1 hour, then filtered, and the resulting solid was washed with water and ethanol to obtain 30.32 g of N'-benzoyl-4-bromobenzoylhydrazide. Under nitrogen protection, 20.00 g of N'-benzoyl-4-bromobenzohydrazide and 250 ml ...
Embodiment 2
[0097]Example 2: Tricarbonyl [1-(4-5'-phenyl-1,3,4-oxadiazolephenyl)-2-(2-pyridyl)benzimidazole] rhenium chloride of the present invention (I), tricarbonyl chloride [1-(4-N-carbazolylphenyl)-2-(2-pyridyl) benzimidazole] rhenium (I), tricarbonyl chloride [N-(4 -5'-phenyl-1,3,4-oxadiazolephenyl)-2,2'-dipyridylamine] rhenium (I) and tricarbonyl chloride [N-(4-N-carbazolyl Fluorescent Characterization of Phenyl)-2,2'-Bipyridylamine]Rhenium(I)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com