Two fluoric dianhydride monomers and preparation method thereof
A dianhydride monomer and monomer technology, applied in the field of dianhydride monomer and its preparation of hyperbranched polyimide, can solve the problems of solvent waste, large amount of solvent, low solid content, etc., and achieve easy operation , Improve light transmittance, simple synthesis route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
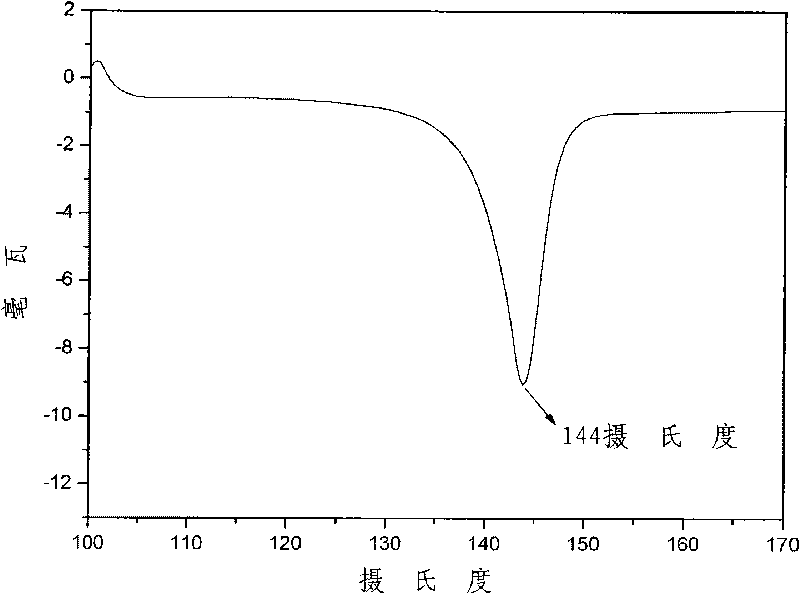
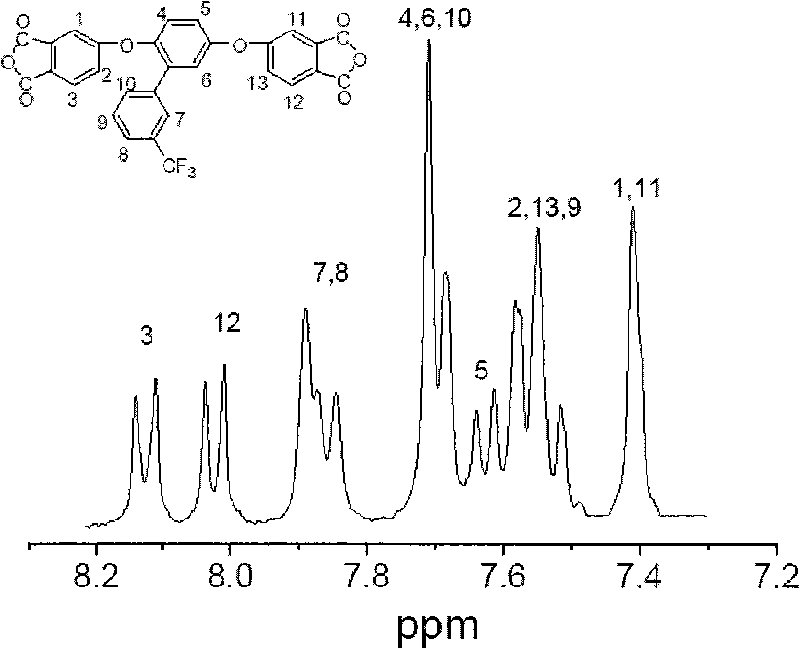
[0039] Embodiment 1: the process of synthesizing 4,4'-(2-(3'-trifluoromethyl-phenyl)-1,4-phenoxy)-phthalic anhydride
[0040]In a 500ml three-neck flask equipped with mechanical stirring, a nitrogen port with a thermometer, and a spherical condenser, add 30.30 grams (0.12mol) of 2-(3'-trifluoromethyl-phenyl)-1,4-terephthalene Phenol, 42.56 grams (0.30mol) of 4-nitrophthalonitrile, 49.68 grams (0.36mol) of potassium carbonate, 240ml N, N-dimethylacetamide (solid content is 51.1%), nitrogen flow, start stirring , reacted for two days, then slowly raised the temperature to 50-80°C, reacted for 2 hours, stopped heating, and continued to stir for a period of time, and stopped stirring after returning to room temperature to end the reaction. The output is in deionized water; then the product is washed repeatedly with deionized water until the water is colorless, filtered by suction, and dried in vacuum to obtain a yellow powder, namely 4,4'-(2-(3'-trifluoromethyl -55.86 g of -pheny...
Embodiment 2
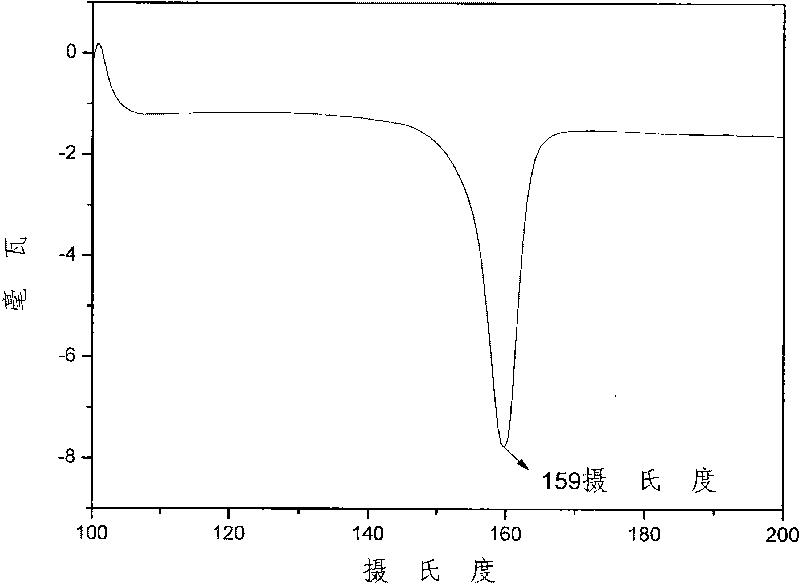
[0046] The method is the same as in Example 1, and the amount of 2-(3', 5'-bistrifluoromethyl-phenyl)-1,4-hydroquinone is changed to 38.40 grams (0.12mol), 42.56 grams (0.3mol ) 4-nitrophthalonitrile, 49.68 grams (0.36mol) of potassium carbonate, 240ml N, N-dimethylacetamide (solid content is 54.4%) is packed into the nitrogen port with thermometer, mechanically stirred , in a 500ml three-neck flask with a spherical condenser, blow nitrogen, start stirring, react for two days, then slowly raise the temperature to 50-80°C, react for 2 hours, stop heating, continue stirring for a while, stop stirring when the room temperature is restored, and end reaction. The material is discharged in deionized water; then the product is washed repeatedly with deionized water until the water is colorless, filtered by suction, and dried in vacuum to obtain a yellow powder, namely 4,4'-(2-(3',5'-di 62.40 g of trifluoromethyl-phenyl)-1,4-phenoxy)-1,1',2,2'-phthalonitrile.
[0047] Put the above ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com