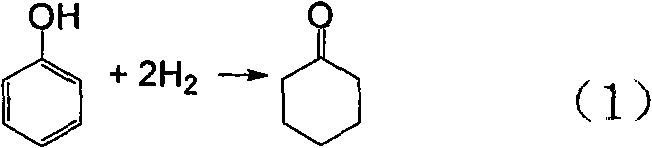
Method and special catalyst for preparing cyclohexanone in one step by phenol hydrogenation
A technology for the preparation of hydrogen and cyclohexanone, which is applied to catalyst supports, metal/metal oxide/metal hydroxide catalysts, physical/chemical process catalysts, etc., to achieve high selectivity, good stability, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1, the molar ratio is 5: 1 loading on TiO 2 AlCl on 3 One-step hydrogenation of phenol to cyclohexanone over a supported Pd catalyst
[0026] Add 1.0mmol phenol, catalyst (by 0.1mmol AlCl 3 (loaded on TiO 2 above) and 0.02 mmol of any of the following supported Pd catalysts: Pd / C, Pd / SiO 2 , Pd / Al 2 o 3 and Pd / SBA-15) and 1ml of dichloromethane, heated to 30°C, fed hydrogen until the system pressure was 1MPa, under stirring conditions, reacted for 30 hours, cooled, degassed, and filtered to separate the catalyst from the reaction solution , and the reaction solution was analyzed by gas chromatography. The gas chromatography conditions are as follows: Agilent 4890D gas chromatograph, FID detector, capillary column (Innowax, 30m × 0.252mm × 0.25μm), take the temperature program, the initial column temperature is 60 ° C, keep for 5 minutes, and then 20 ° C / The heating rate of min was increased to 200°C for 5 minutes. The carrier gas is 99.99% high-puri...
Embodiment 2
[0030] Embodiment 2, the molar ratio is 5: 1 load on SiO 2 AlCl on 3 One-step hydrogenation of phenol to cyclohexanone over a supported Pd catalyst
[0031] Add 1.0mmol phenol, catalyst (by 0.1mmol AlCl 3 (loaded on SiO 2 above) and 0.02 mmol of any of the following supported Pd catalysts: Pd / C, Pd / SiO 2 , Pd / Al 2 o 3 and Pd / SBA-15) and 1ml of dichloromethane, heated to 30°C, fed hydrogen until the system pressure was 1MPa, under stirring conditions, reacted for 30 hours, cooled, degassed, and filtered to separate the catalyst from the reaction solution , and the reaction solution was analyzed by gas chromatography. The gas chromatography conditions are the same as in Example 1.
[0032] The gas chromatographic analysis results of three repeated experiments are shown in Table 2:
[0033] Table 2. Made of AlCl 3 Results of hydrogenation of phenol catalyzed by catalysts composed of different supported Pd catalysts
[0034] catalyst
Embodiment 3
[0035] Embodiment 3, the molar ratio is 5: 1 load on Al 2 o 3 AlCl on 3 One-step hydrogenation of phenol to cyclohexanone over a supported Pd catalyst
[0036] One-step Preparation of Cyclohexanone by Hydrogenation of Phenol
[0037] Add 1.0mmol phenol, catalyst (by 0.1mmol AlCl 3 (loaded at Al 2 o 3 above) and 0.02 mmol of any of the following supported Pd catalysts: Pd / C, Pd / SiO 2 , Pd / Al 2 o 3 and Pd / SBA-15) and 1ml of dichloromethane, heated to 50°C, fed hydrogen until the system pressure was 1MPa, under stirring conditions, reacted for 30 hours, cooled, degassed, and filtered to separate the catalyst from the reaction solution , and the reaction solution was analyzed by gas chromatography. The gas chromatography conditions are the same as in Example 1. The gas chromatographic analysis results of three repeated experiments are shown in Table 3:
[0038] The gas chromatographic analysis results of three repeated experiments are shown in Table 3:
[0039] Table 3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com