Method for preparing targeted curcumin nanoparticles for treating ulcerative colitis
A technology of curcumin and nanoparticles, applied in the field of medicine, to achieve the effects of prolonging half-life, improving burst release, and good freeze-drying and reconstitution performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0023] Preparation Example 1: Preparation of Curcumin PLGA Nanoparticles
[0024] Table 1 The composition of the prescription of curcumin PLGA nanoparticles
[0025]
[0026] Preparation:
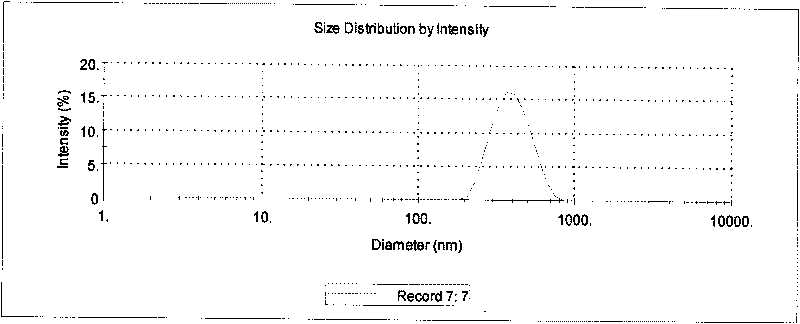
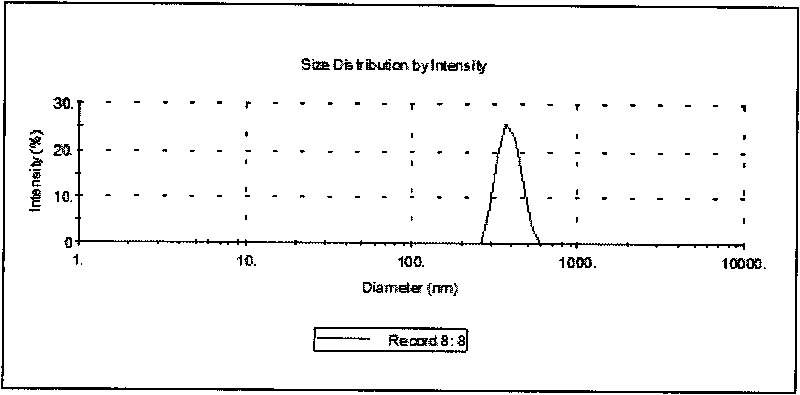
[0027] Co-dissolve the PLGA and curcumin in the above prescription amount in dichloromethane to obtain the organic phase. The prepared PVA solution is the water phase. Pour the organic phase into the water phase, stir at 15000rpm for 2-3min, and place it in a probe-type ultrasonic cell. In the crushing instrument, 400W ultrasonic 60-240 times, then take out and carry out low-temperature high-speed centrifugation 10000rpm-20000rpm, collect curcumin PLGA nanoparticles ( figure 1 ), freeze-dried after redispersing with 2%-10% sucrose solution to obtain curcumin PLGA nanoparticles ( figure 2 ).
preparation Embodiment 2
[0028] Preparation Example 2: Particle Size Measurement of Nanoparticles
[0029] The particle size and distribution of nanoparticles were measured by laser particle size analyzer. The measured particle size is 409±11nm, which is a normal distribution of effective diameter.
preparation Embodiment 3
[0030] Preparation Example 3: Determination of Nanoparticle Drug Loading Rate
[0031] Weigh the freeze-dried powder of nanoparticles, add a certain amount of acetone to dissolve it, measure the content of curcumin at 425nm, and calculate the drug loading rate and encapsulation efficiency of nanoparticles.
[0032] Table 2 Drug loading rate and encapsulation efficiency of curcumin PLGA nanoparticles
[0033]
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com