Prulifloxacin composition and preparation method thereof, and synthesis method of raw material drugs
A technology of prulifloxacin and its composition, which is applied in the field of medicine, can solve the problems of affecting the safety of medication, the lack of strictness of pharmaceutical excipients, intestinal stimulation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0098] prescription
[0099] Prulifloxacin 132.1g
[0100] Lactose 40g
[0101] Hydroxypropyl Cellulose 9.5g
[0103] Povidone K30 18g
[0104] Sodium Carboxymethyl Starch 6g (tablet)
[0105] Make 1000 capsules
[0106] Described prulifloxacin can be a commercially available product or can be prepared according to the following method:
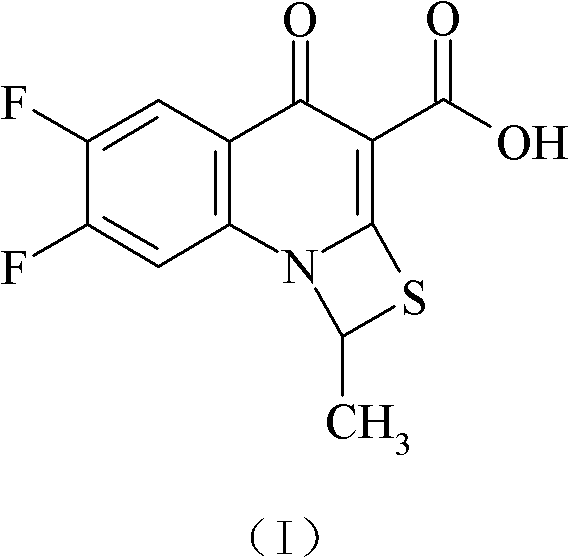
[0107] (1) 6,7-difluoro-1-methyl-4-oxo-4H-[1,3]thiazetidin[3,2-a]quinoline-3-carboxylic acid (I )Synthesis:
[0108] With 6,7-difluoro-1-methyl-4-oxo-4H-[1,3]thiazetidin[3,2-a]quinoline-3-carboxylic acid ethyl ester 100g and 645ml of glacial acetic acid, 970ml of concentrated hydrochloric acid, and 640ml of water were reacted at 72°C for 12 hours, concentrated under reduced pressure, diluted with 16.13 times of water, filtered and washed until the pH was neutral, and dried to obtain 88.5g of compound (I). 97.3%, elemental analysis C 12 h 7 f 2 NO 3 S, calculated value C 50.88%, H 2.4...
Embodiment 2
[0118] prescription
[0119] Prulifloxacin 132.1g
[0120] Lactose 40g
[0121] Hydroxypropyl Cellulose 9.5g
[0123] Povidone K30 18g
[0124] Sodium Carboxymethyl Starch 6g (tablet)
[0125] Make 1000 capsules
[0126] Described prulifloxacin can be a commercially available product or can be prepared according to the following method:
[0127] (1) 6,7-difluoro 1-methyl-4-oxo-4H-[1,3]thiazetidin[3,2-a]quinoline-3-carboxylic acid (I) Synthesis:
[0128] With 6,7-difluoro-1-methyl-4-oxo-4H-[1,3]thiazetidin[3,2-a]quinoline-3-carboxylic acid ethyl ester 100g and 645ml of glacial acetic acid, 970ml of concentrated hydrochloric acid, and 640ml of water were reacted at 68°C for 12 hours, concentrated under reduced pressure, diluted with 16.13 times of water, filtered and washed until the pH was neutral, and dried to obtain 89.0g of compound (I). 97.8%;
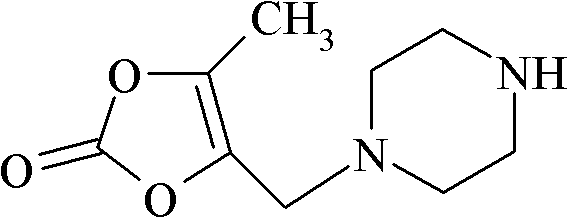
[0129] (2) Synthesis of 5-methyl-4-piperazinemethyl-1,3-dioxolenedione (I...
Embodiment 3
[0138] prescription
[0139] Prulifloxacin 132.1g
[0140] Lactose 40g
[0141] Hydroxypropyl Cellulose 9.5g
[0143] Povidone K30 18g
[0144] Sodium Carboxymethyl Starch 6g (tablet)
[0145] Make 1000 capsules
[0146] Described prulifloxacin can be a commercially available product or can be prepared according to the following method:
[0147] (1) 6,7-difluoro-1-methyl-4-oxo-4H-[1,3]thiazetidin[3,2-a]quinoline-3-carboxylic acid (I )Synthesis:
[0148] With 6,7-difluoro-1-methyl-4-oxo-4H-[1,3]thiazetidin[3,2-a]quinoline-3-carboxylic acid ethyl ester 10kg and 64.5L of glacial acetic acid, 9.7L of concentrated hydrochloric acid, and 6.4L of water were reacted at 76°C for 12 hours, concentrated under reduced pressure, diluted with 16.13 times of water, filtered and washed until the pH was neutral, and dried to obtain 8.87kg of compound (I) , yield 97.5%;
[0149] (2) Synthesis of 5-methyl-4-piperazinemethyl-1,3-dioxolenedi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com