Method for synthesizing optical enantiomer 6-fluoro-3,4-dihydro-2H-1-benzopyran-2-carboxylic acid and 6-fluoro-3,4-dihydro-2H-1-benzopyran-2-carboxylate
A technology of benzopyran and a synthesis method, applied in directions such as organic chemistry, can solve the problems of high cost of enantiomeric separation, inability to localize raw materials, difficulty in mass production of products, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
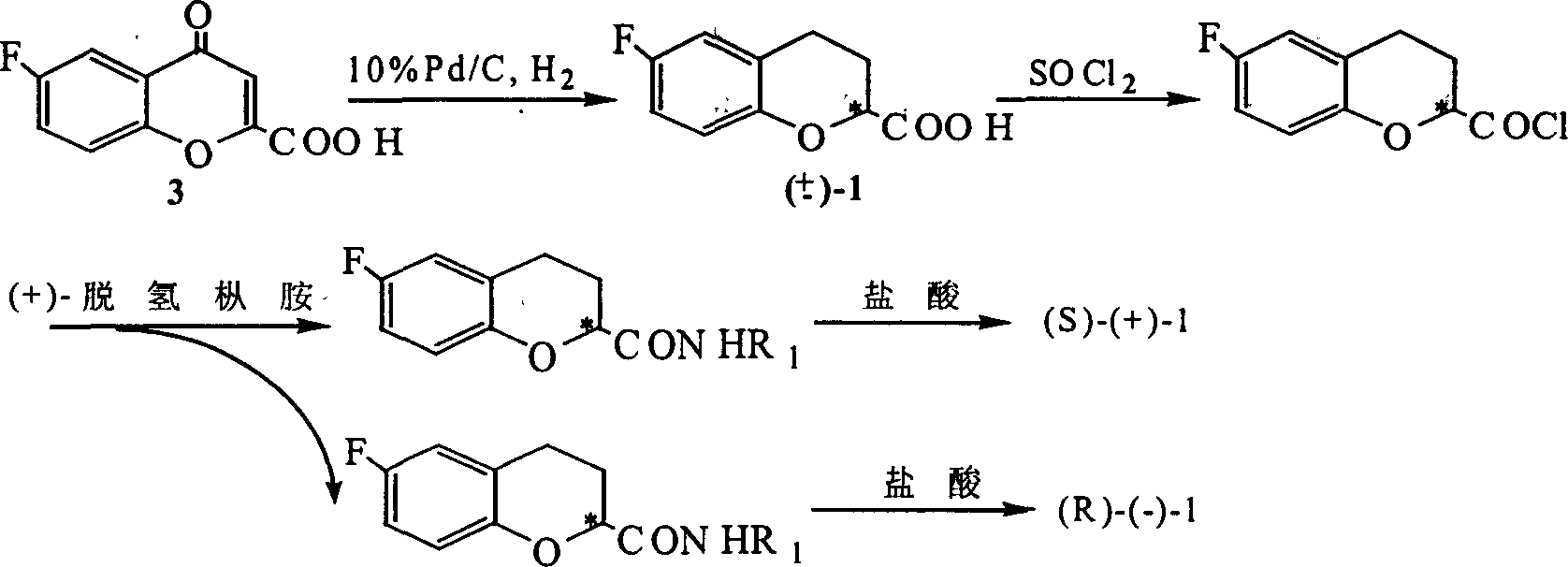
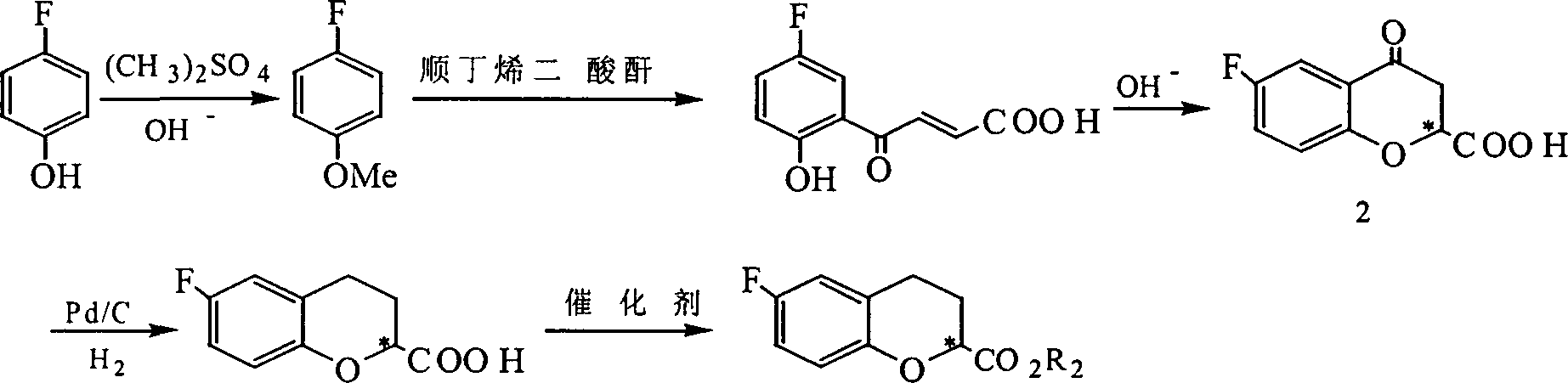
[0052]Example 1. Preparation of (±)-6-fluoro-3,4-dihydro-2H-1-benzopyran-2-carboxylic acid [wherein (±)-1] and (±) -6-fluoro-3,4-dihydro-2H-1-benzopyran-2-carboxylate [wherein (±)-1b]
[0053] 1.1 p-Fluoroanisole
[0054] Dissolve p-fluorophenol (25.6g, 0.05mol) in 500ml of 5wt% NaOH aqueous solution, add dropwise dimethyl sulfate (32g, 0.254mol), heat to 70°C, stop stirring for 5 hours, and wait for the system to cool to room temperature Finally, extract with ethyl acetate 20ml×3, collect the ethyl acetate layer, dry with anhydrous sodium sulfate, filter, and remove the solvent by rotary evaporation to obtain a light yellow viscous liquid, which is distilled under reduced pressure and collected at 38-40°C / The 5mmHg fraction gave p-fluoroanisole (6.2g, 98%).
[0055] 1.2 4-(5-fluoro-2-hydroxy)phenyl-4-keto-2,3-butenoic acid
[0056] Feed maleic anhydride (4g, 0.04mol), aluminum trichloride catalyst (15g, 0.112mol), p-fluoroanisole (2.5g, 0.02mol), dissolve in 50ml of carbo...
Embodiment 2
[0066] 1.1 p-Fluoroanisole
[0067] Dissolve p-fluorophenol (51.2 g, 0.1 mol) in 5 wt% Na 2 CO 3 Add dimethyl sulfate (64g, 0.508mol) dropwise to 1000ml of aqueous solution, heat to 75°C, stop stirring for 5 hours, after the system is cooled to room temperature, extract with ethyl acetate 40ml×3, collect the ethyl acetate layer, Dry with anhydrous sodium sulfate, filter, and remove the solvent by rotary evaporation to obtain a light yellow viscous liquid, distill under reduced pressure, collect fractions at 38-40°C / 5mmHg to obtain p-fluoroanisole (12.4g, 98%) .
[0068] 1.2 4-(5-fluoro-2-hydroxy)phenyl-4-keto-2,3-butenoic acid
[0069] Feed with maleic anhydride (8g, 0.08mol), catalyst aluminum trichloride (30g, 0.224mol), p-fluoroanisole (5.0g, 0.04mol), dissolve in 55ml of dichloromethane, heat to 60°C, React for 2 hours. After the reaction is completed, add ice water and hydrochloric acid mixture to the reaction system, and vigorously stir. 4-(5-fluoro-2-hydroxy)phenyl...
Embodiment 3
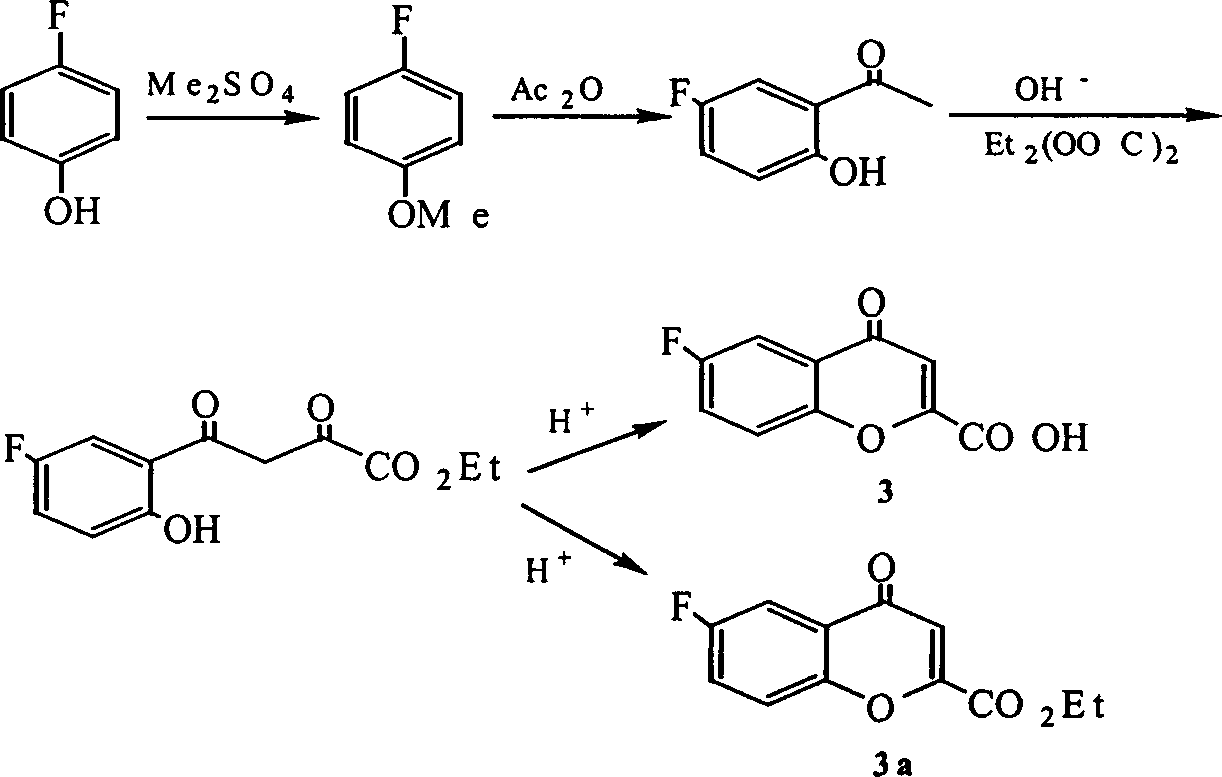
[0079] Prepare (±)-6-fluoro-3,4-dihydro-2H-1-benzopyran-2-carboxylic acid [wherein (±)-1] and (±)-6-fluoro -3,4-dihydro-2H-1-benzopyran-2-carboxylate [wherein (±)-1b]
[0080] 1.1 p-Fluoroanisole
[0081] The preparation of p-fluoroanisole is the same as in Example 1.
[0082] 1.2 5-fluoro-2-hydroxy-acetophenone
[0083] p-Fluoroanisole (0.1mol), CS 2 100ml, catalyst aluminum trichloride (0.25mol), slowly add acetic anhydride (0.12mol) dropwise under stirring, the reaction temperature is 75°C, after the reaction is finished, add an appropriate amount of hydrochloric acid and crushed ice to the system, stir vigorously, and divide The organic layer was taken out, the aqueous layer was extracted with ethyl acetate (3 × 25ml), the combined organic layers were washed with water, and finally the organic layer was washed with Na 2 SO 4 After drying and removing the solvent, the product 5-fluoro-2-hydroxy-acetophenone was obtained. Mp55~56℃.
[0084] 1.3 (a) 5-fluoro-2-hydroxy-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com