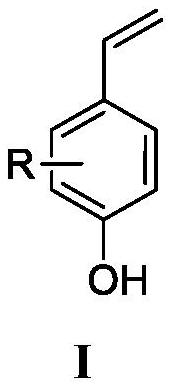
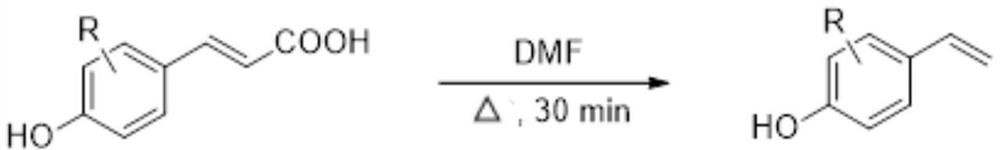
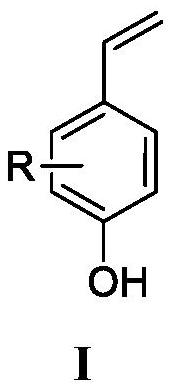
Preparation method of 4-vinylphenol compound
A vinylphenol and compound technology, applied in the field of preparation of 4-vinylphenol compounds, can solve problems such as limited universality of substrates, and achieve the effects of stable yield, improved production efficiency and short reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1: a kind of preparation method of 4-vinylphenol compound:
[0024] Add 0.2mmol of p-hydroxycinnamic acid and 1mL of solvent DMF to the pressure-resistant reaction bottle, heat and stir in an oil bath at 200°C for 30 minutes; after the reaction, extract the reaction solution with ethyl acetate three times, and wash the organic layer with water and saturated sodium chloride solution in sequence , then dry the washed organic layer with anhydrous sodium sulfate, filter, and the residue after the filtrate is concentrated under reduced pressure is subjected to silica gel column chromatography (V 石油醚 / V 乙酸乙酯 =5:1) separation and purification to obtain 4-vinylphenol compound I-1 with a yield of 89%.
[0025] The nuclear magnetic resonance (NMR) of the prepared 4-vinylphenol compound (I-1) 1 H NMR and 13 C NMR) detection data are: 1 H NMR (600MHz, DMSO-d6) δ7.29–7.23 (m, 2H), 6.72 (d, J = 8.5Hz, 2H), 6.60 (dd, J = 17.6, 10.9Hz, 1H), 5.57 (dd, J=17.6,0.9Hz,1H),5....
Embodiment 2
[0026] Embodiment 2: a kind of preparation method of 4-vinylphenol compound:
[0027] Add 0.2mmol of 3,4-dihydroxycinnamic acid and 1mL of solvent DMF to the pressure-resistant reaction flask, heat and stir in an oil bath at 200°C for 30min; The organic layer was washed with solution, and the organic layer after washing was dried with anhydrous sodium sulfate, filtered, and the residue after the filtrate was concentrated under reduced pressure was subjected to silica gel column chromatography (V 石油醚 / V 乙酸乙酯 =5:1) separation and purification to obtain 4-vinylphenol compound I-2 with a yield of 87%.
[0028] The nuclear magnetic resonance (NMR) of the prepared 4-vinylphenol compound (I-2) 1 H NMR and 13 C NMR) detection data are: 1 H NMR (600MHz, CD 3 OD) δ6.94(d, J=1.8Hz, 1H), 6.78(dd, J=8.1, 1.8Hz, 1H), 6.75(d, J=8.1Hz, 1H), 6.61(dd, J=17.6, 10.9Hz,1H),5.56(dd,J=17.6,0.9Hz,1H),5.06(dd,J=10.9,0.8Hz,1H). 13 C NMR (151MHz, CD 3 OD)δ145.16, 144.98, 136.75, 130.03, 118.31, ...
Embodiment 3
[0029]Embodiment 3: a kind of preparation method of 4-vinylphenol compound:
[0030] Add 0.2mmol of 3-methoxy-4-hydroxycinnamic acid and 1mL of solvent DMF to a pressure-resistant reaction flask, heat and stir in an oil bath at 200°C for 30min; Wash the organic layer with sodium chloride solution, then dry the washed organic layer with anhydrous sodium sulfate, filter, and the residue after the filtrate is concentrated under reduced pressure is subjected to silica gel column chromatography (V 石油醚 / V 乙酸乙酯 =5:1) separation and purification to obtain 4-vinylphenol compound I-3 with a yield of 94%.
[0031] The nuclear magnetic resonance (NMR) of the prepared 4-vinylphenol compound (I-3) 1 H NMR and 13 C NMR) detection data are: 1 H NMR (600MHz, CDCl 3 )δ6.95–6.91(m,2H),6.87(d,J=8.1Hz,1H),6.64(dd,J=17.5,10.8Hz,1H),5.65(s,1H),5.59(d,J =17.5Hz,1H),5.13(d,J=10.9Hz,1H),3.91(s,3H). 13 CNMR (151MHz, CDCl 3 )δ146.59, 145.64, 136.63, 130.28, 120.08, 114.35, 111.47, 108.01, 55.89. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com