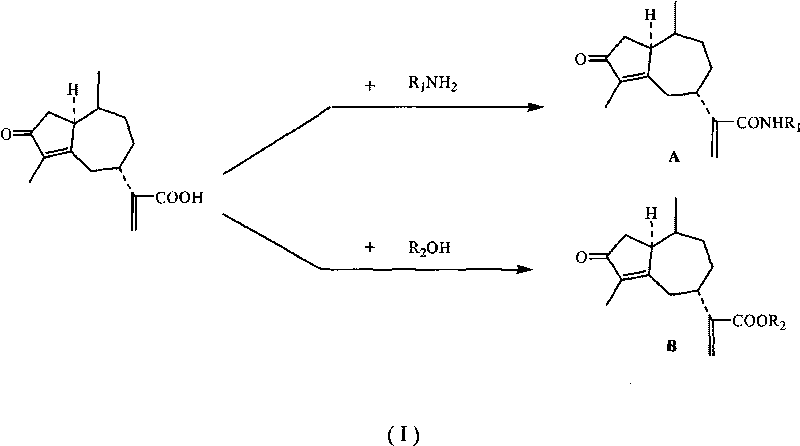
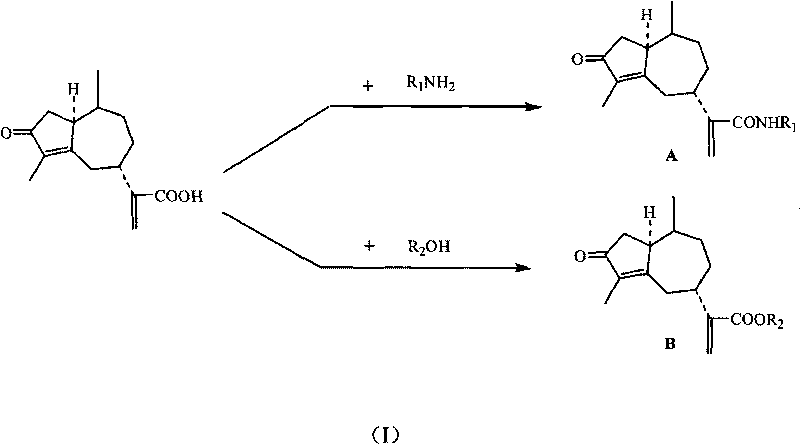
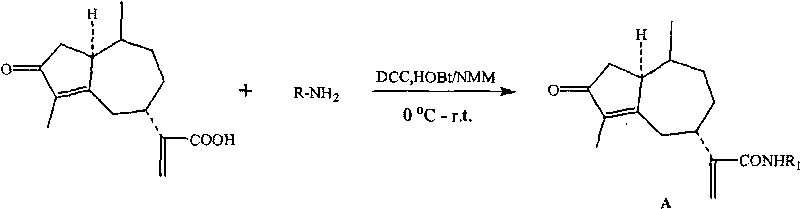Derivates of rupestonic acid and uses thereof
A technology of artemisinic acid and derivatives, which is applied in the field of artemisinic acid derivatives, and can solve the problems of high toxicity and side effects and high drug activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] Preparation of Artemisinic Acid
[0075] Artemisinic acid is extracted by a conventional continuous extraction method. Take 5 kg of crushed Artemisia annua, extract it three times with 1 liter of 95% ethanol, combine the extracts, extract concentrated extracts, and extract the extracts with ethyl acetate for multiple times . The ethyl acetate layers were combined, concentrated and separated on a silica gel column to obtain a crude product of artemisinic acid, which was recrystallized to obtain a pure product of artemisinic acid as colorless rod-shaped crystals, mp.132-134°C.
[0076] IR(KBr)υ: 3230, 2970-2860, 1720, 1680, 1635, 1415, 1390, 1238, 958cm -1 ; 1 HNMR (600MHz, CDCl 3 ): 0.67 (d, J=7.2Hz, 3H, CH 3 ), 1.63(m, 1H), 1.64(m, 1H), 1.81(m, 1H), 1.84(m, 1H), 1.88(m, 1H), 2.06(m, 1H), 2.14(m, 1H) , 2.46(m, 1H), 2.64(m, 1H), 2.86(m, 1H), 2.90(m, 1H), 3.22(m, 1H), 5.76(s, 1H), 6.40(s, 1H); 13 CNMR (150MHz, CDCl 3 ): 7.9, 12.0, 31.4, 35.1, 36.4, 37.5, 38.2, 41.1,...
Embodiment 2
[0087] According to Example 1, a branch of artemisinic acid was prepared;
[0088] Preparation of Class B Synthesis of Artemisinic Acid Derivatives (Taking the Synthesis of Artemisinic Acid (P-Methyl-Phenyl)-Ester as an Example)
[0089] Add 0.124g (0.5mmol) of artemisinic acid and 0.113g (0.55mmol) of DCC into a 25mL round-bottomed flask, add 8mL of dry THF, stir under ice cooling, and add 0.031g (0.25mmol) of DMAP to the reaction system. After reacting in ice bath for 30 minutes, add 0.128g (0.60mmol) of p-cresol to the reaction system, stir and react in ice bath for 30 minutes, then naturally rise to room temperature and react for 8 hours, filter out the precipitated DCU, concentrate the filtrate and separate by column to obtain the target Compound B-10. The remaining B-type compounds were synthesized according to the synthesis method of B-10. The synthesized compounds were passed through IR, 1 HNMR, ESI-MS and other analytical methods were characterized. Some of these ...
Embodiment 3
[0103] According to Example 1, a branch of artemisinic acid was prepared;
[0104] Preparation of an artemisinic acid amide derivative (A compound) inorganic acid salt or organic acid salt:
[0105] (1) Preparation of N(2-bromo-phenyl)-artemisinic acid amide (A-5) hydrochloride
[0106] Add 0.5mmol of N(2-bromo-phenyl)-artemisinic acid amide into 20mL of 5% hydrochloric acid aqueous solution, stir slightly to dissolve it, add an appropriate amount of ethanol, refrigerate to crystallize, filter, and dry in vacuo to obtain N (2-Bromo-phenyl)-artemisinic acid amide hydrochloride, yield: 66%.
[0107] (2) Preparation of N(2-bromo-phenyl)-artemisinic acid amide (A-5) acetate
[0108] Add 0.5mmol A-5 into a 50mL single-necked round-bottomed flask containing 10mL of dichloromethane, add 2mL of glacial acetic acid, stir at 30-40°C for 1-2h, refrigerate and crystallize after cooling, filter, and dry in vacuo to obtain A-5 vinegar Salt, the yield is about 58%.
[0109] Salts of Clas...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com