Method of inducing differentiation into myocardial cells using g-csf
A technique for cardiomyocytes and differentiation inducers, applied in the field of cardiomyocytes and differentiation inducers of cardiomyocytes, can solve the problems of low efficiency of cardiomyocyte induction and no reports pointing out G-CSF cardiomyocyte differentiation induction.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
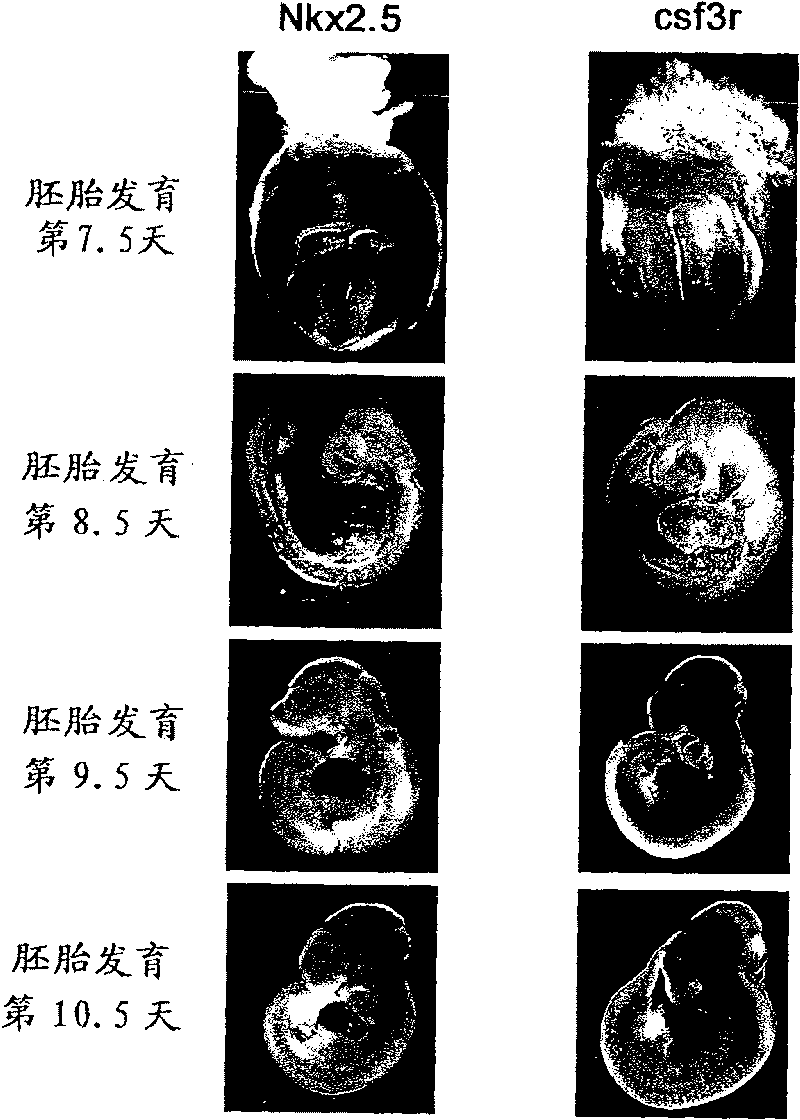
[0117] Example 1: Expression of G-CSF receptor in myocardium derived from mouse embryonic heart
[0118] The expression of G-CSF receptor in the myocardium derived from mouse embryonic heart was investigated by the following method.
[0119] (1) Research by in situ hybridization
[0120]Pregnant ICR wild-type mice were purchased from CLEA, Japan. Embryos were removed at days 7.5, 8.5, 9.5, and 10.5 of embryonic development, and using a digoxigenin-labeled RNA probe, as described in the literature (Sasaki H et al., Development 118, 47-59 (1993) ) for whole-mount in situ hybridization (WISH) of the heart. The full-length cDNAs of mouse G-CSF receptor (csf3r) and cardiac-specific transcription factor Nkx2.5 (accession numbers NM_008711, NM_008700, respectively) were obtained by reverse transcription PCR (RT-PCR) and subcloned into pBluescript plasmid middle. Use 5'-CCC CTC AAA CCT ATC CTG CCT C-3' (SEQ ID NO: 2) as the sense primer of csf3r, and 5'-TCC AGG CAG AGA TCA GCG AAT...
Embodiment 2
[0125] Example 2: In vivo role of G-CSF in cardiogenesis (1)
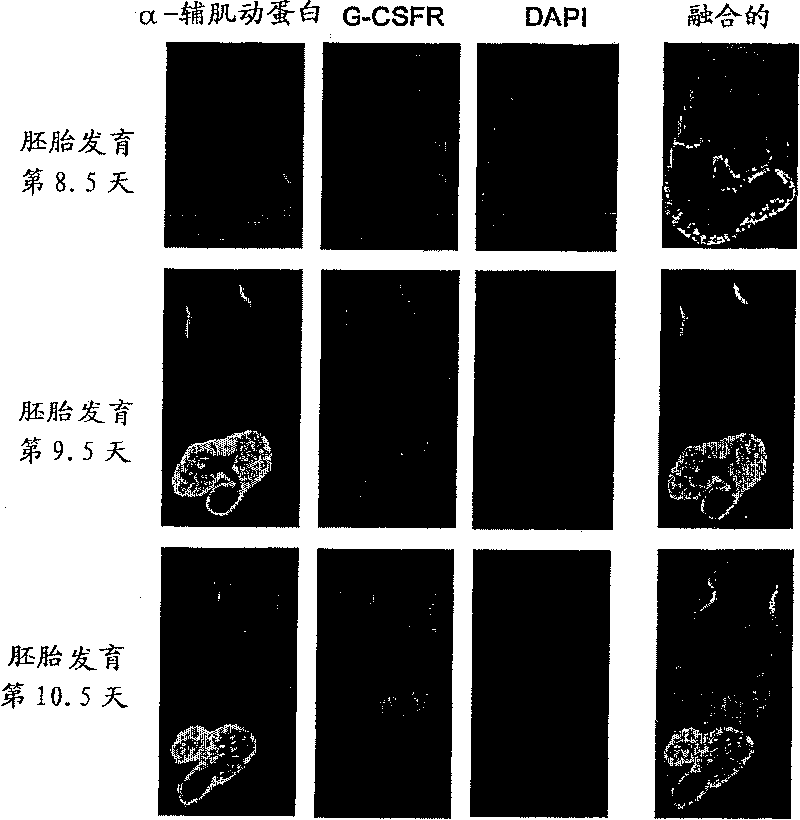
[0126] The mice were opened on pregnancy day 9.0, and G-CSF (100 ng / kg) or PBS (phosphate-buffered saline) as a control was directly administered in utero. BrdU (bromodeoxyuridine) was then intraperitoneally administered to the mother on day 9.5 of pregnancy. BrdU is taken up during DNA synthesis and proliferation can be assessed by immunostaining. Embryos were removed on day 12.5 of gestation to make heart slices. Test with the same method as the immunostaining described in embodiment 1 (2), its result is shown in Figure 4 . It was confirmed that G-CSF promotes myocardial proliferation in embryos.
[0127] In addition, heart sections were stained with hematoxylin and eosin, and the results of microscopic observation are shown in Figure 5 a. In the hearts of G-CSF-administered embryos, elongation of the trabecular layer was observed.
[0128] Furthermore, G-CSFR knockout mice lacking the G-CSF receptor (he...
Embodiment 3
[0132] Example 3: In vivo role of G-CSF in cardiogenesis (2)
[0133] On the 9.0th day of gestation, 2 ng was directly administered into the uterus of pregnant mouse mothers. Embryos were removed on day 13.5 of pregnancy, heart sections were made, and immunostained with Phospho-Histon H3. Phospho-Histon H3 is a pigment that is specifically stained during cell proliferation. The results obtained are shown in Figure 7 . G-CS administration was observed to promote myocardial proliferation in embryos.
[0134] The labeling index was calculated by the following formula.
[0135] Labeling index = Phospho-Histon H3 positive nuclei / total nuclei × 100 (%)
[0136] The results obtained are shown in Figure 8 . G-CSF was observed to strongly enhance myocardial proliferation from embryonic day 8.5 to day 10.5.
[0137] In addition, in order to study the effect of G-CSF on the apoptosis of the embryonic heart, Tunel staining was performed on the heart on the 10.5th day of embryoni...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com