Insulin slow release micron sphere composition and preparation method thereof
A technology of composition and insulin, which is applied in the directions of pharmaceutical formulations, medical preparations of non-active ingredients, bulk delivery, etc., can solve the problems of inability to prepare nano-insulin, and achieve a smooth and round surface, no adhesion of particles, and regular particles Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
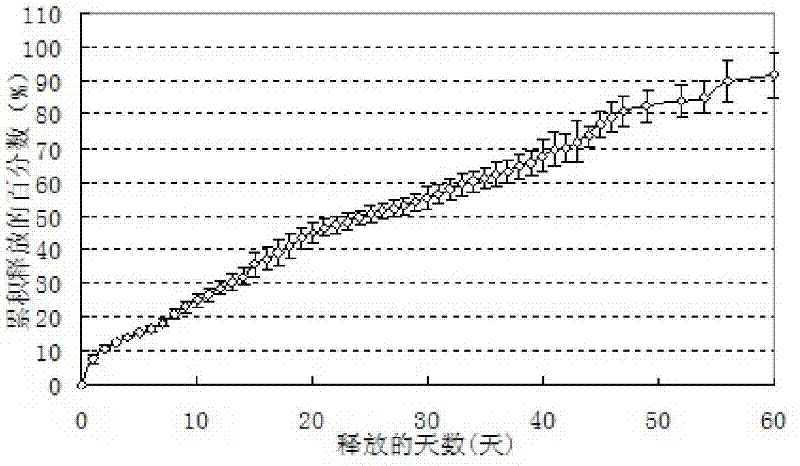
Examples
Embodiment 1
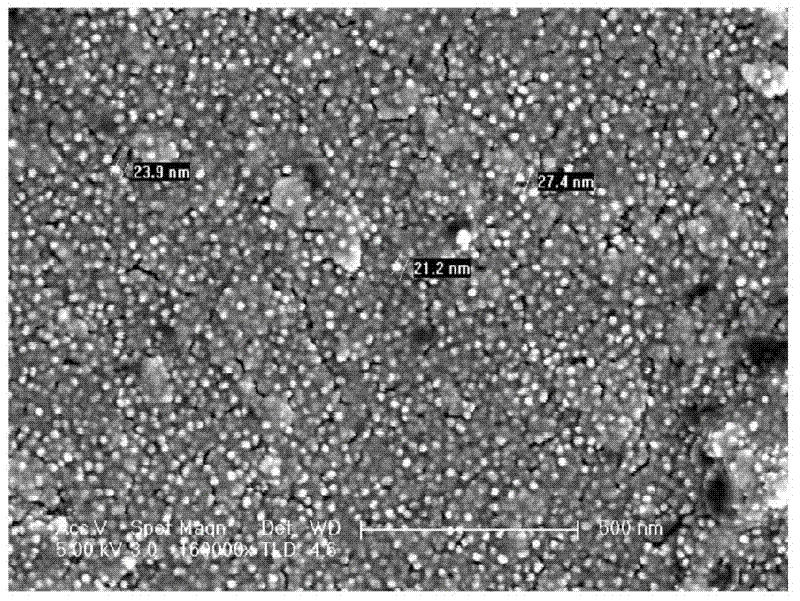
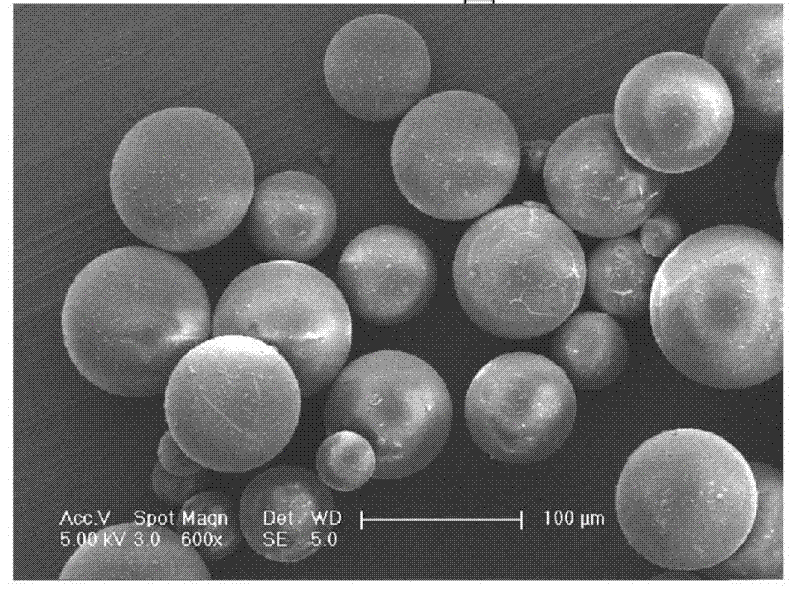
[0038] ① Preparation of insulin nanosphere composition
[0039] a) dissolving zinc acetate in water in advance, and preparing solutions with concentrations of 0.001%, 0.001%, 10%, or 20% by weight;
[0040] b) dissolving insulin in hydrochloric acid solution (pH<2) with a weight percent concentration of 1%, 10%, 20% or 40% PEG (molecular weight of 8000 Daltons) to prepare a weight percent concentration of 0.001% , 1%, 10% or 20% etc.;
[0041] c) then add the solution of step a) dropwise to the solution of b) in the solution of the above steps a) and b) according to the weight ratio of 1:1, 1:10, 1:3 or 1:4 etc. and continuously to stir;
[0042] d) then pre-freeze the mixture of the above step c) in the freezer for 8-32 hours, and then freeze-dry,
[0043] e) Dissolving the PEG in the freeze-dried powder of the above step d) with an organic solvent, centrifuging to remove the supernatant, i.e. the organic solution of PEG, repeating three times, then evaporating to dryness,...
Embodiment 2
[0051] ① Preparation of insulin nanosphere composition
[0052] a) Dissolving zinc sulfate in advance in dextran solutions with a molecular weight of 10,000 at a weight concentration of 0%, 0.001%, 5%, 10% or 20%, and preparing the concentration by weight to be 0.001%, 0.001%, 0.001%, 5%, 10%, or 20% solutions;
[0053] b) dissolving insulin in acidic (hydrochloric acid solution, pH < 2) weight percentage concentration: 1%, 5%, 10%, 20% or 40% PEG (molecular weight is 6000 Dalton) to prepare weight percentage concentration They are: 0.001%, 1%, 5%, 10% or 20%, etc.;
[0054] c) Then add the solution of step a) dropwise to the solution of step b) according to the weight ratio of 1:1, 1:10, 1:8, 1:3 or 3:14, etc. solution with constant stirring;
[0055] d) then pre-freeze the mixture of the above step c) in the freezer for 8-32 hours, and then freeze-dry,
[0056] e) Dissolving the PEG in the freeze-dried powder of the above step d) with an organic solvent, centrifuging to ...
Embodiment 3
[0064] ① Preparation of insulin nanosphere composition
[0065] a) Dissolving zinc nitrate in advance in dextran solutions with a molecular weight of 100,000 at a weight concentration of 0%, 0.001%, 5%, 10% or 20%, and preparing the concentration of 0.001%, 0.001%, 0.001%, 5%, 10%, or 20% solutions;
[0066] b) dissolving insulin in acidic (hydrochloric acid solution, pH < 2) weight percentage concentration: 1%, 5%, 10%, 20% or 40% PEG (molecular weight is 4000 Dalton) to prepare weight percentage concentration They are: 0.001%, 1%, 5%, 10% or 20%, etc.;
[0067] c) Then add the solution of step a) dropwise to the solution of step b) according to the weight ratio of 1:1, 1:10, 1:8, 1:3 or 3:14, etc. solution with constant stirring;
[0068] d) then pre-freeze the mixture of the above step c) in the freezer for 8-32 hours, and then freeze-dry,
[0069] e) Dissolving the PEG in the freeze-dried powder of the above step d) with an organic solvent, centrifuging to remove the s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com