Method for synthesizing (7-methoxy-1-naphthyl) acetonitrile
A synthesis method and a methoxyl technology are applied in chemical instruments and methods, preparation of carboxylic acid nitrile, preparation of organic compounds, etc., and can solve the problems of high cost of raw materials, high toxicity of methylene chloride, dark color, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
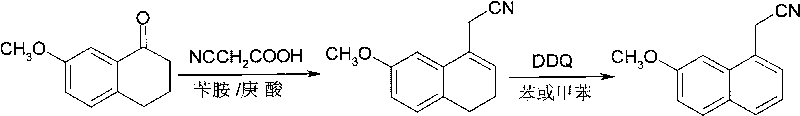
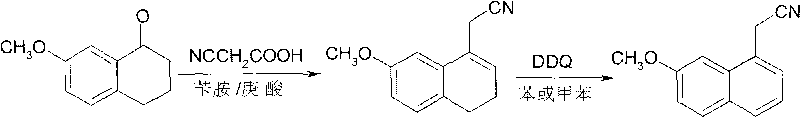
[0026] (1) Dehydration acetonitrile: put 211.2kg of 7-methoxytetralin-1-one, 153.6kg of cyanoacetic acid, 32.4kg of benzylamine, 40kg of heptanoic acid, and 1200kg of toluene into a 3000L reaction kettle. The temperature was raised to 115°C to reflux, and the reflux reaction was divided into water for 24 hours. After cooling, a solution of (7-methoxy-3,4-dihydro-1-naphthyl)acetonitrile was obtained.
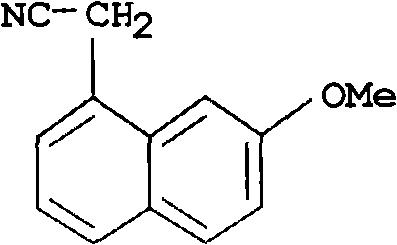
[0027] (2) Dehydroaromatization: 300 kg of DDQ was dissolved in 400 kg of toluene solution, and the solution was added dropwise to the reaction liquid obtained in step (1), the reaction temperature was 25° C., and the reaction was stirred for 2.0 h. The reaction solution was filtered, and the filtrate was washed with alkali, water and saturated brine. After the solvent was removed by distillation under reduced pressure, the residue was recrystallized through ethanol: water (V / V=8: 2) to obtain 222.5kg white to pale yellow crystalline solid (7-methoxy-1-naphthyl) acetonitrile, yi...
Embodiment 2
[0029] (1) Dehydration acetonitrile: Put 176kg of 7-methoxytetralin-1-one, 128kg of cyanoacetic acid, 27kg of benzylamine, 33kg of heptanoic acid, and 1500kg of benzene into a 3000L reaction kettle. The temperature was raised to 86°C to reflux, and the reflux reaction was divided into water for 40 hours. After cooling, a solution of (7-methoxy-3,4-dihydro-1-naphthyl)acetonitrile was obtained.
[0030] (2) Dehydroaromatization: 250 kg of DDQ was dissolved in 400 kg of benzene solution, and the solution was added dropwise to the reaction solution obtained in step (1), the reaction temperature was 45° C., and the reaction time was 1.0 h. The reaction solution was filtered, and the filtrate was washed with alkali, water and saturated brine. After the solvent was evaporated by distillation under reduced pressure, the residue was recrystallized through ethanol: water (V / V=8: 2) to obtain 177kg of white to light yellow crystalline solid (7-methoxy-1-naphthyl) acetonitrile, yield 90 ...
Embodiment 3
[0032] (1) Dehydration acetonitrile: put 176kg of 7-methoxytetralin-1-one, 102kg of cyanoacetic acid, 21.1kg of benzylamine, 26.4kg of heptanoic acid, and 920kg of toluene into a 2000L reaction kettle. The temperature was raised to 116°C to reflux, and the reflux reaction was divided into water for 30 hours. After cooling, a solution of (7-methoxy-3,4-dihydro-1-naphthyl)acetonitrile was obtained.
[0033](2) Dehydroaromatization: 227kg of DDQ was dissolved in 230kg of toluene solution, and the solution was added dropwise to the reaction solution obtained in step (1), the reaction temperature was 0°C, and the reaction time was 5h. The reaction solution was filtered, and the filtrate was washed with alkali, water and saturated brine. After the solvent was evaporated by distillation under reduced pressure, the residue was recrystallized from ethanol: water (V / V=8: 2) to obtain 167kg of white to light yellow crystalline solid (7-methoxy-1-naphthyl) acetonitrile, yield 85 %. The...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com