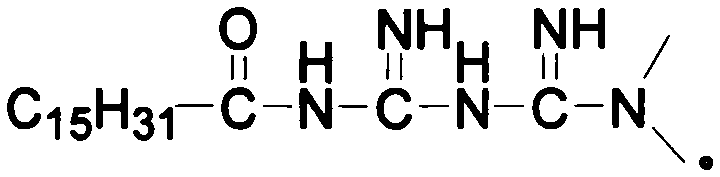Lipid prodrug of guanidinium-containing drug and drug plastid thereof
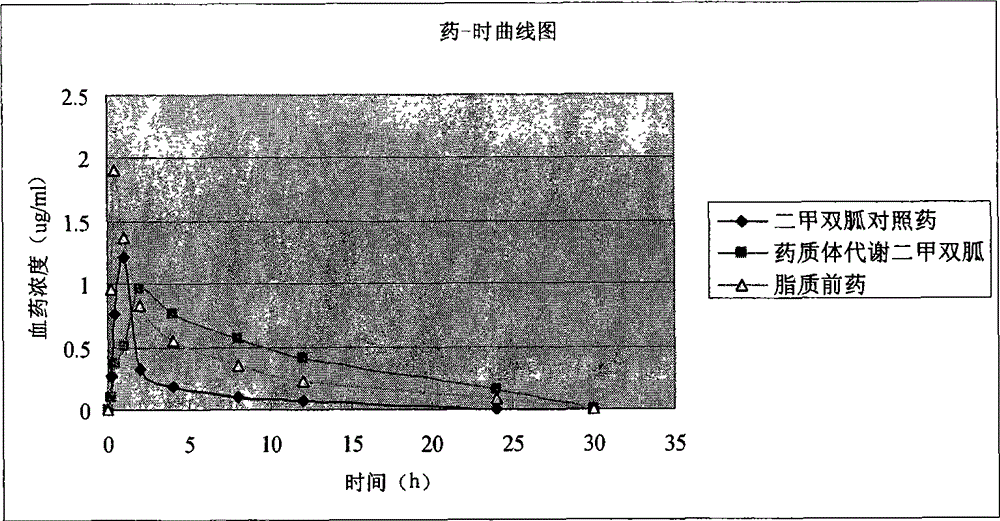
A drug substance and prodrug technology, which is applied in the field of amphiphilic lipid prodrug and its drug substance, can solve the problems of difficult to achieve and low bioavailability, optimize the operation process, and slow down the fluctuation of blood drug concentration , reduce the effect of gastrointestinal irritation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 2
[0023] The preparation of embodiment 1. dipalmitin
[0024]
[0025] Heat and melt the palmitic acid solid (60°C), add excess SOCl dropwise to it 2 , distilled after stirring for several hours, first distilled SOCl at low temperature 2 , Distilled under reduced pressure to obtain colorless palmitoyl chloride, sealed and waterproof.
[0026] When dilute sulfuric acid is added dropwise to hot water dissolved in calcium glycerate, a large amount of white precipitate is formed immediately. After the dropwise addition was completed, the white precipitate was removed by hot filtration under the condition of maintaining the temperature to obtain an aqueous solution of glyceric acid. The water was distilled off under reduced pressure until the mother liquor was a viscous white liquid, and then the heating was stopped. Add anhydrous acetone, shake well to dissolve, add anhydrous magnesium sulfate to dry overnight. The next day, the desiccant was removed by filtration, and the ...
Embodiment 2 2
[0028] Example 2. Synthesis of Dipalmitoyl Glycerate-Metformin
[0029]
[0030] Put 0.58 g (1 mmol) of dipalmitoyl glyceride into a 50 ml flask, add 5 ml of SOCl 2 . Reflux for 8 hours, and the water pump decompresses to remove the generated HCl and SOCl 2 . 5 ml of anhydrous dichloromethane was added thereto, and 0.1 ml of anhydrous pyridine and 0.13 g of metformin were dissolved in 3 ml of methanol. The methanol solution was added dropwise into the reaction flask, stirred at room temperature for 24 hours, refluxed for 4 hours, and cooled. The obtained solution was directly spin-dried, 30 ml of dichloromethane was added, and the insoluble matter was filtered off. Wash twice with 10 mL of water, dry the organic layer and spin dry. Ethyl acetate was added, and 0.45 g of a white solid was precipitated. 1 H-NMR (CDCl 3 ): 0.85(t, 6H), 1.23(s, 48H), 1.52(m, 4H), 2.27(t, 2H), 2.32(t, 2H), 2.50(s, 6H), 4.34(m, 1H), 4.43 (m, 1H), 5.2 (m, 1H).
Embodiment 3
[0031] Example 3. Synthesis of Palmitoyl-Metformin
[0032]
[0033]Metformin hydrochloride (0.33 g, 2 mmol) was added to 2 ml of water and stirred to dissolve, then sodium hydroxide (0.08 g, 2 mmol) was added, and stirred at room temperature for 1 hour. A solution of palmitoyl chloride (0.7 g, 2.5 mmol) in acetone was added dropwise and stirred at room temperature for 2 hours. Add acetone until the precipitate no longer separates out, filter, and spin the filtrate to dryness. Add 5 ml of dichloromethane to dissolve, and filter to remove insoluble matter. Wash twice with 5 mL of water, dry the organic layer and spin dry. Ethyl acetate was added, and 0.48 g of a white solid was precipitated. 1 H-NMR (CDCl 3 ): 0.96(t, 3H), 1.31(s, 24H), 1.57(m, 2H), 2.18(t, 2H), 2.47(s, 6H).
PUM
| Property | Measurement | Unit |
|---|---|---|
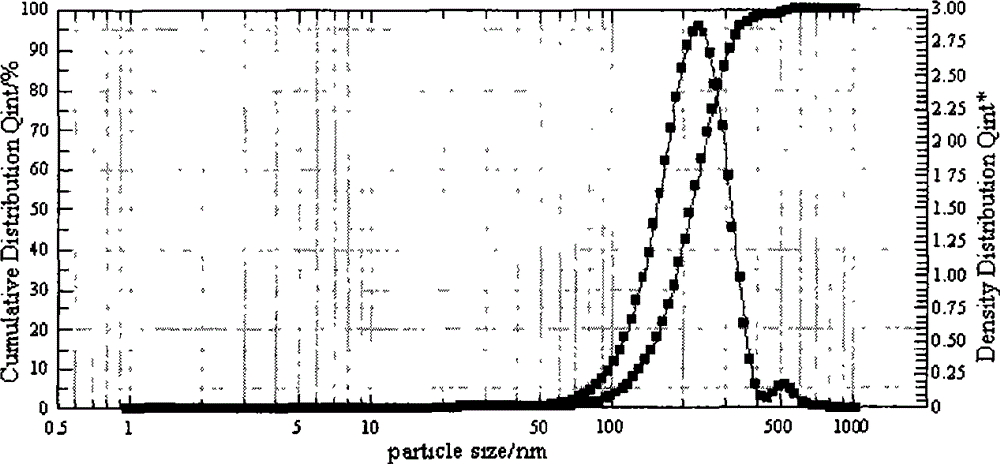
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com