Carbazole compound, organic electroluminescence device containing carbazole compound and preparation method thereof
An electroluminescent device, an organic technology, applied in chemical instruments and methods, electrical solid-state devices, semiconductor/solid-state device manufacturing, etc., can solve problems such as low maximum brightness, low efficiency, unbalanced electron and hole concentrations in the light-emitting layer, etc. , to achieve the effect of improving performance and efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
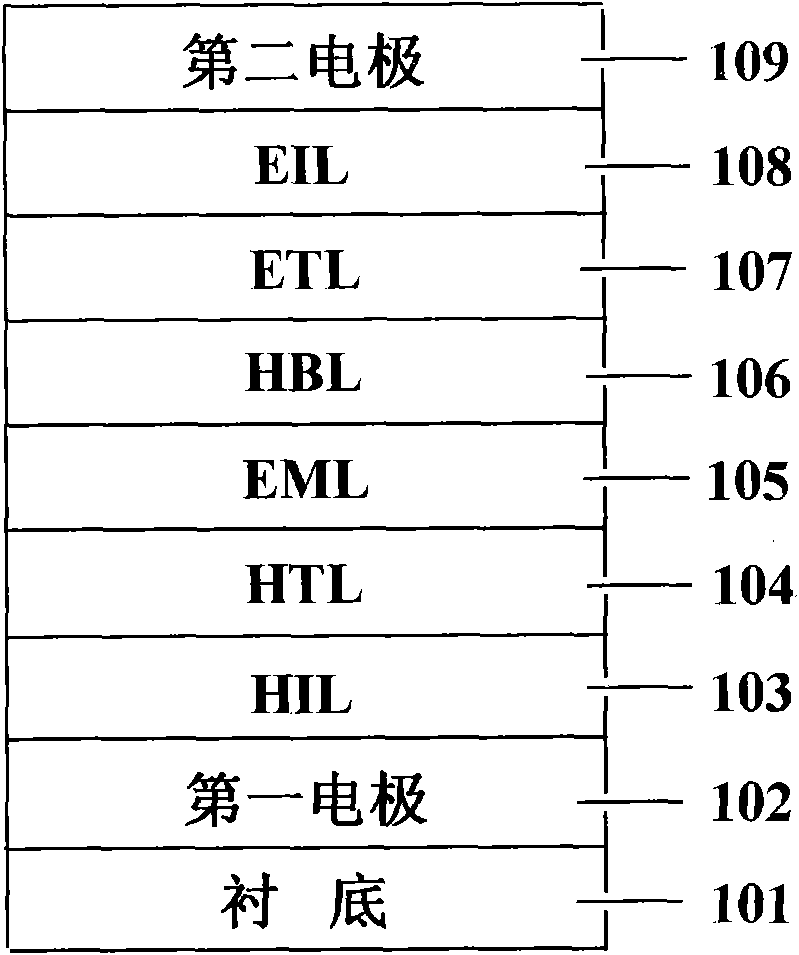
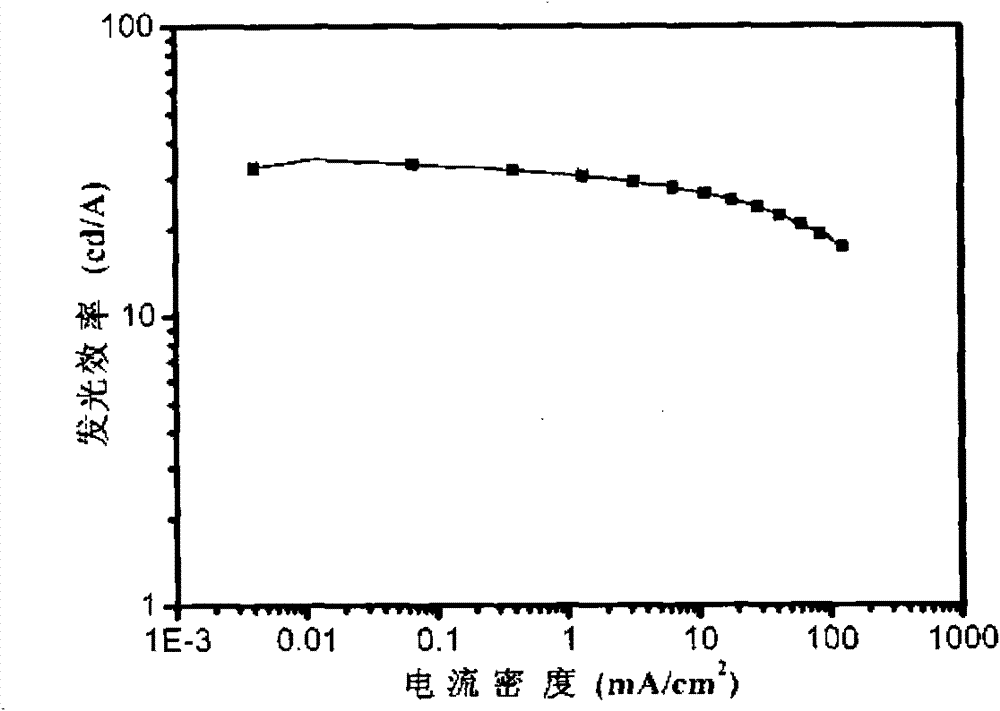
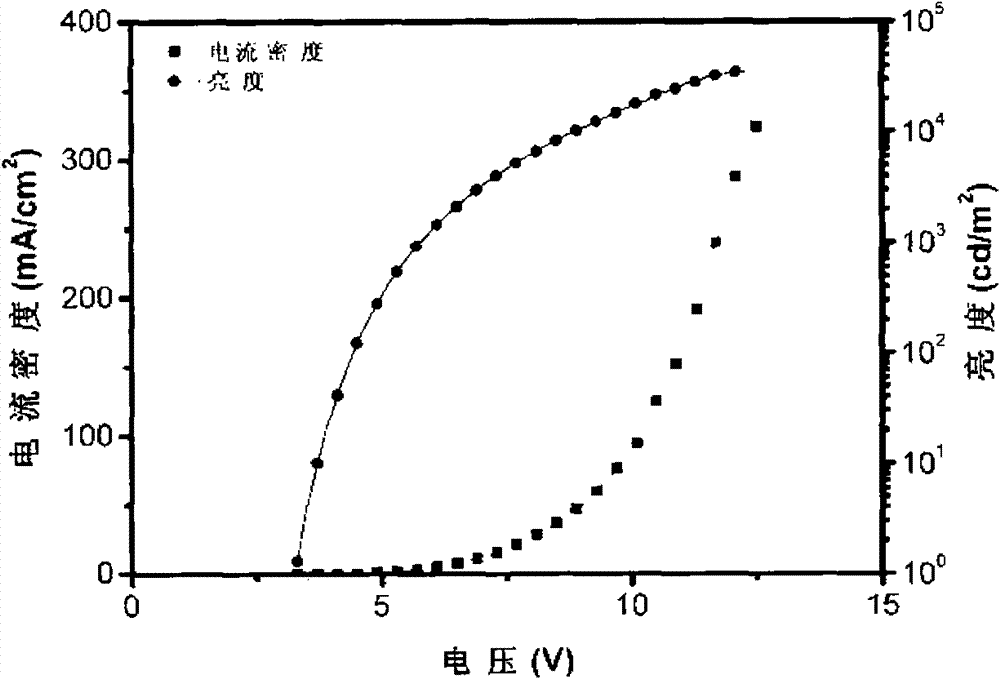
Image
Examples
preparation example Construction
[0055] The present invention does not have special limitation to the preparation method of CzBPO, preferably comprises the following steps:
[0056] Using 4,4'-dibromobiphenyl and carbazole as raw materials, potassium carbonate and CuI as catalysts, 1,3-dimethyl-2-imidazolidinone (DMI) as reaction medium, and argon as protection Gas, react under the condition of 100°C-250°C to obtain N-(4'-bromobiphenyl)-N-carbazole;
[0057] With N-(4′-bromobiphenyl)-N-carbazole and diphenylphosphorous chloride (Ph 2 PCl) as raw material, with tetrahydrofuran (THF) as the reaction medium, with n-BuLi as the initiator, react under room temperature conditions, the reaction product obtained with H 2 o 2 Oxidation gives the product.
[0058] The product is subjected to nuclear magnetic resonance analysis, and its proton nuclear magnetic resonance spectrum proves that the product is indeed CzBPO having the structure of formula (I-a).
[0059] According to the present invention, when Ar 1 Pref...
Embodiment 1
[0095] Add 75mmol (23g) 4,4'-dibromobiphenyl, 50mmol (8.3g) carbazole, 100mmol (14g) potassium carbonate, 10mmol (2.0g) CuI and 10mL 1,3-dimethyl-2 - Imidazolidinone (DMI), under the protection of argon, heated to 190 ° C for 20 hours; after cooling, the reaction mixture was dissolved in dichloromethane, washed three times with water, dried with anhydrous sodium sulfate, then filtered, concentrated, silica gel Obtain 7.9gN-(4'-bromobiphenyl)-N-carbazole after column separation, productive rate 40.2%, through characterization analysis, confirm that this product is N-(4'-bromobiphenyl)-N-carbazole azole. 1 H NMR (300MHz, CDCl 3 )[ppm]: δ8.16(d, J=7.8Hz, 2H), 7.78(d, J=8.7Hz, 2H), 7.62-7.66(m, 4H), 7.55(d, J=8.7Hz, 2H ), 7.40-7.48 (m, 4H), 7.31 (td, J=7.2, 1.5Hz, 2H);
[0096] Add 3mmol (1.2g) N-(4′-bromobiphenyl)-N-carbazole and 50mL dry tetrahydrofuran (THF) into the dry reaction flask, and cool to -78 ℃, add 4.5mmol n-BuLi (1.8mL 2.5M n-hexane solution) into the reaction f...
Embodiment 2
[0101] Add 200mmol (4.8g) of magnesium chips and a small amount of iodine to the dry reaction flask, add 10mL of dry THF under the protection of argon, slowly add 150mL of THF solution containing 200mmol (47.2g) of p-dibromobenzene, and react at 50°C for 4 hours , add 90mmol (16.1g) phenyl phosphorus dichloride (PhPCl 2 ), continue to react for 24 hours. Slowly add 20mL H 2 o 2 After stirring for 2 hours, the reaction mixture was poured into water, extracted with dichloromethane, washed three times with water, dried with anhydrous sodium carbonate, filtered, concentrated, and separated on a silica gel column to obtain 22.6g of bis(4-bromophenyl)phenylphosphine , The yield was 57.7%. After characterization and analysis, it was confirmed that the product was indeed bis(4-bromophenyl)phenylphosphine. 1 H NMR (300MHz, CDCl 3 )[ppm]: δ7.58-7.68 (m, 7H), 7.48-7.55 (m, 6H).
[0102] Add 0.5mmol (0.22g) bis(4-bromophenyl) phenylphosphine, 3mmol (0.50g) carbazole, 3mmol (0.29g) t-...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com