Novel acyclic nucleoside phosphonate predrug
A prodrug and a new type of technology, applied in the field of anti-hepatitis B and anti-HIV drugs, can solve the problems of oxidative pathway metabolism, low carnitine level, toxicity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
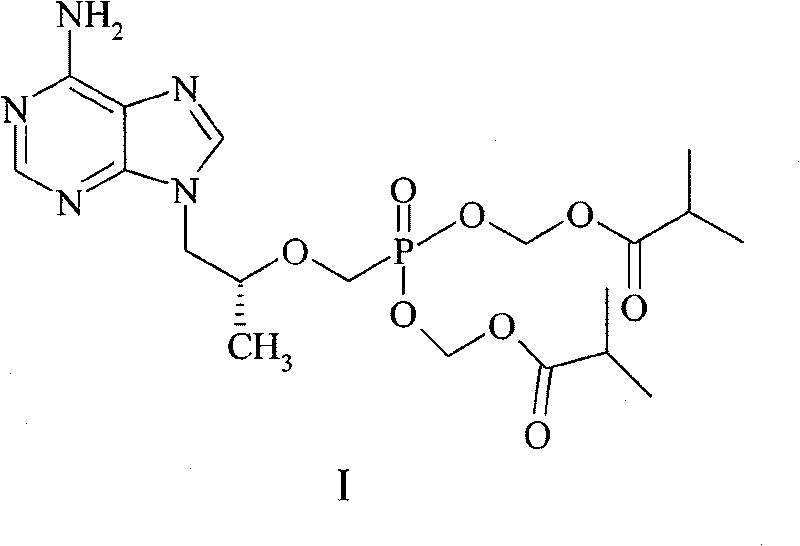
[0015] The preparation of embodiment 1 compound I
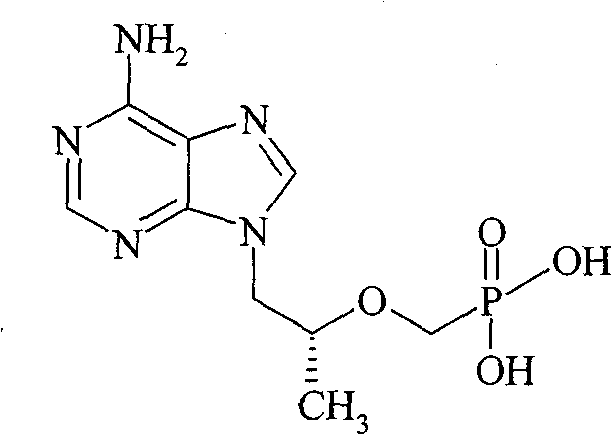
[0016] 20 g of R-PMPA, 100 ml of N-methylpyrrolidone, 50 ml of triethylamine, and 80 ml of chloromethyl isobutyrate were sequentially added to the reaction flask, and the reaction was stirred at 60° C. for 2.5 hours. Add 200ml of ethyl acetate at room temperature, stir for 10min, filter, add 100ml of water, shake and separate the liquids, extract the aqueous phase with 100ml of ethyl acetate, combine the organic phases, wash with 150ml of water, and dry the organic phase over anhydrous sodium sulfate. Filter, concentrate to about 150ml volume, add 6.7g oxalic acid dihydrate, stir for 3h. Filter, wash twice with a small amount of ethyl acetate, drain, transfer the resulting solid to a 500ml Erlenmeyer flask, add 300ml of water, and heat in a water bath at 55°C to dissolve. Then slowly add saturated aqueous sodium bicarbonate solution at room temperature to adjust the pH to about 7, extract twice with dichloromethane, 200ml ea...
Embodiment 2
[0017] The preparation of the fumarate of embodiment 2 compound I
[0018] Dissolve 10 g of compound I in 100 ml of isopropanol, add 2.5 g of fumaric acid, heat in a water bath at 50°C to dissolve, and stir at room temperature. After full crystallization, it was filtered, washed twice with ethyl acetate, and dried under reduced pressure at 45°C to obtain 8.0 g of white solid. Yield 65%.
[0019] 1 H-NMR (d 6 -DMSO, ppm): 1.03~1.08(m, 15H), 2.52~2.59(m, 2H), 3.88~3.98(m, 3H), 4.13~4.17(m, 1H), 4.23~4.27(m, 1H) , 5.47~5.56 (m, 4H), 6.62 (s, 2H), 7.27 (s, 2H), 8.03 (s, 1H), 8.13 (s, 1H). ESI-MS: 488.1, 510.2.
Embodiment 3
[0020] The preparation of the L-tartrate of embodiment 3 compound I
[0021] Dissolve 12.7g of compound I in 100ml of acetone, add 3.7g of L-tartaric acid-200ml of acetone solution, and stir at room temperature. After fully analyzing the crystallization, it was filtered, washed twice with an appropriate amount of acetone, and dried under reduced pressure at 45°C to obtain 11.4 g of a white solid. Yield 69%.
[0022] 1 H-NMR (d 6 -acetone, ppm): 1.15~1.23(m, 15H), 2.60~2.65(m, 2H), 3.88~4.10(m, 3H), 4.25~4.44(m, 2H), 4.60(s, 2H), 5.59 ~5.68 (m, 4H), 7.08 (s, 2H), 8.13 (s, 1H), 8.24 (s, 1H). ESI-MS: 488.2, 510.2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com