Preparation method of alkyl arabinoside
A technology of arabinoside and arabinose, applied in the field of preparation of alkyl arabinoside, can solve the problems of difficult separation of product and catalyst, deepened product color, complicated operation steps, etc., and achieves the effects of low production cost, convenient operation and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
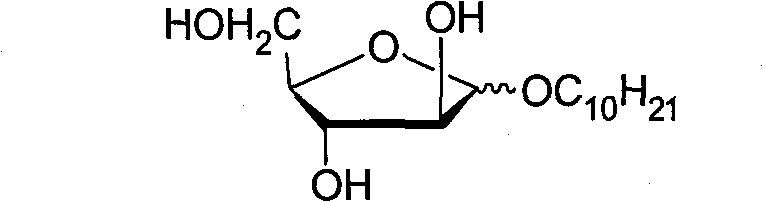
[0017] a. Synthesis of fully acylated decyl arabinoside
[0018] Take 10g of fully acylated arabinose, mix 6.0ml of n-decyl alcohol (Acros) and 100ml of anhydrous dichloromethane, stir at 0°C and add 3.0mL of trimethylsilyl trifluoromethanesulfonate (TMSOTf) (Aldrich) , then heated to 25°C and continued to stir, and carried out the full acylation reaction for 3 hours. After the reaction, separated by column chromatography to obtain 5.99 g of pure white solid, which was full acylated decanyl arabinoside, and its yield was 45%. (α:β≈1.7:1).
[0019] NMR structure analysis of fully acylated decanyl arabinoside was carried out, and the test data are as follows:
[0020] 1 H NMR (500MHz, CDCl 3 ), δ: 0.88(t, 3H, CH 3 ), 1.20-1.32(m, 14H), 1.54-1.60(m, 2H), 2.03, 2.07, 2.14(s, 9H, 3COCH 3 ), 3.46(m, 1H), 3.63(m, 1H), 3.86(m, 1H), 4.03(m, 1H), 4.40(m, 1H), 5.03(m, 1H), 5.20(m, 1H) , 5.26 (brs 1H).
[0021] b. Synthesis of Decyl Arabinoside
[0022] Take 2g (4.8mmol) of the ab...
Embodiment 2
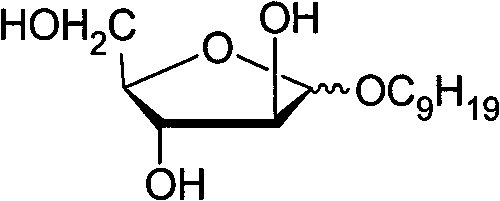
[0029] a. Synthesis of fully acylated nonyl arabinoside
[0030] Mix 1 g of fully acylated arabinose, 0.6 ml of nonanol (Acros, 97%) and 40 ml of anhydrous toluene, stir at room temperature and add 1.0 mL of boron trifluoride ether (chemically pure), then heat to 50°C and continue stirring. The full acylation reaction was carried out for 5 hours. After the reaction, the pure white solid was separated by column chromatography to obtain 0.45 g of full acylated nonyl arabinoside, and the yield was 35% (α:β>19:1).
[0031] b, the synthesis of nonyl arabinoside
[0032] Take the above-mentioned fully acylated nonyl arabinoside 0.45g (1.1mmol) and add 1.5ml of anhydrous methanol to mix, stir slowly at room temperature and add 0.12mol L·L -1 MeONa / MeOH solution 0.3ml, carry out deacylation reaction 1 hour, drop Dowex-50 resin (H + , ~5g) neutralized to pH=7, then filtered, and the filtrate was suspended and evaporated under reduced pressure to remove methanol, and the white pure so...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com