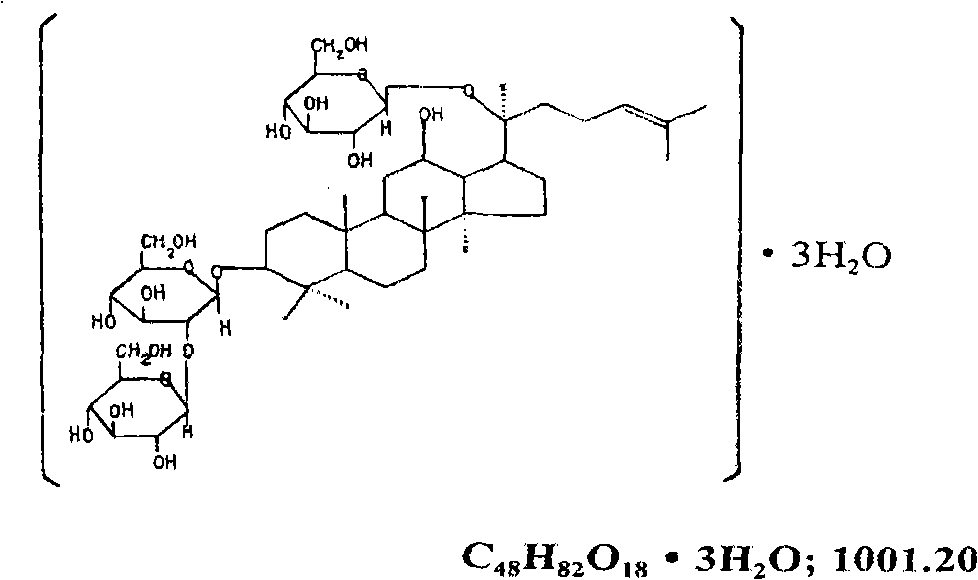
Preparation for panaxoside-Rd aqueous solution of propylene glycol and new application thereof in inflammation resistance, immune suppression and organ transplant rejection resistance
A technology of ginsenoside and propylene glycol, which is applied in anti-inflammatory agents, plant raw materials, medical preparations of non-active ingredients, etc., and can solve the problem of low content of ginsenoside-Rd
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0088] Embodiment 1. The formula research of the low concentration ginsenoside-Rd solution of propylene glycol dissolving
[0089] Objective: To adjust the concentration of propylene glycol so that ginsenoside-Rd can be completely dissolved, in order to obtain a true solution, so as to ensure the safety of clinical medication.
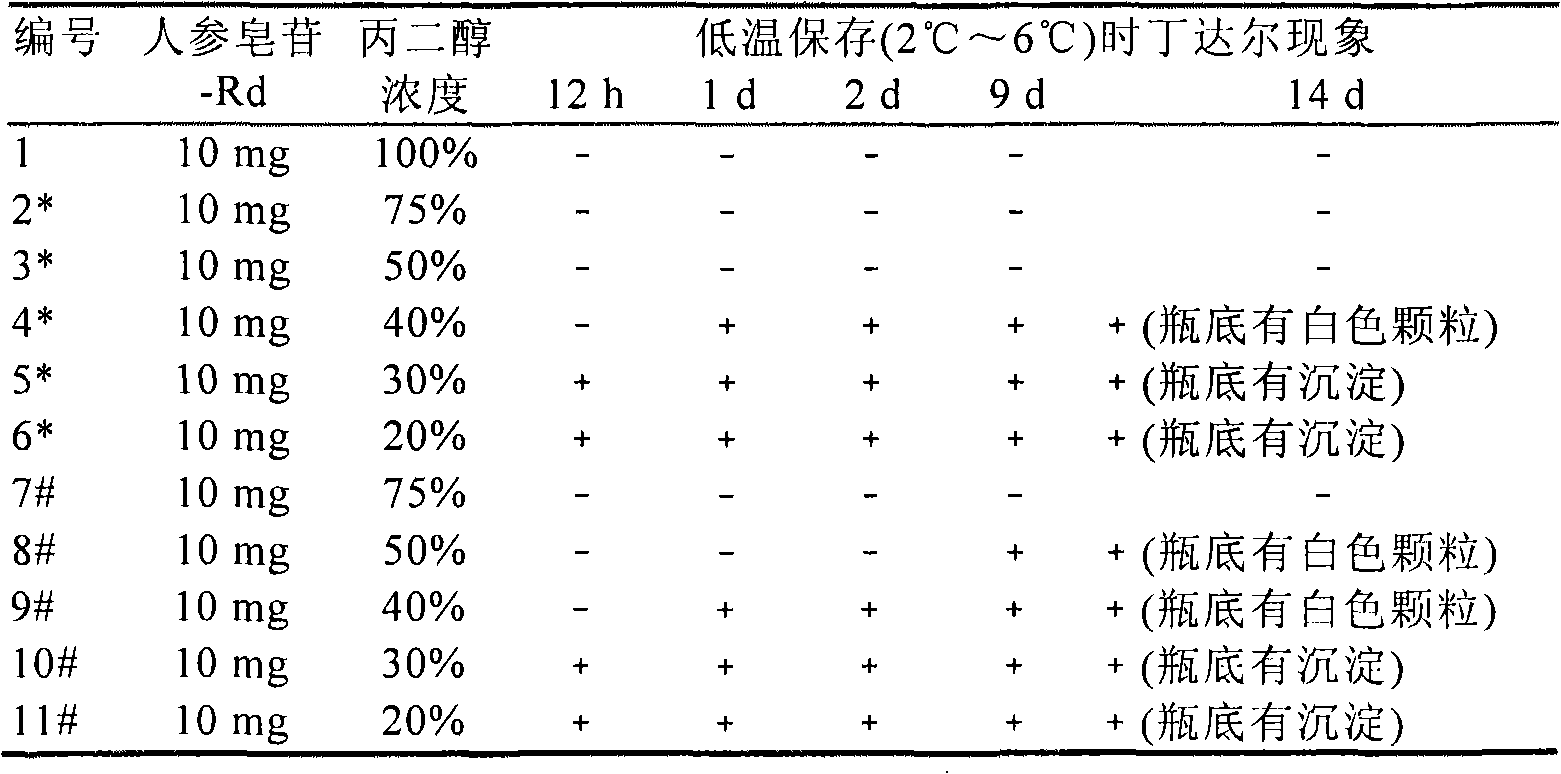
[0090] Method: select different volume ratio (20 volume %, 30 volume %, 40 volume %, 50 volume %, 55 volume %, 60 volume %, 75 volume % and 100 volume %) propylene glycol aqueous solution as solvent, with 10mg / ml and 5mg Add ginsenoside-Rd at the content of / ml to determine the propylene glycol concentration that can fully dissolve ginsenoside-Rd. After obtaining the true solution, examine the dissolution state of the medicinal solution under freezing or refrigerated conditions. The available preparations are diluted with water for injection and then added to 250ml of normal saline for mixing or directly diluted with normal saline to investigate the d...
Embodiment 2
[0140] Embodiment 2. The impact of high concentration propylene glycol on the solubility of high concentration ginsenoside-Rd
[0141] Objective: To increase the concentration of propylene glycol, increase the content of ginsenoside-Rd in turn, and investigate the effect of the concentration of propylene glycol on the solubility of ginsenoside-Rd.
[0142]Method: select propylene glycol with a concentration ≥ 50% by volume, add ginsenoside-Rd with different contents (20mg / ml, 25mg / ml, 50mg / ml, 75mg / ml) respectively [dissolve ginsenoside-Rd powder with pure propylene glycol before Add quantitative water (Method B)]. Place it at room temperature for several days, put the sample in a good solution state in refrigerator or freezer, observe the solution state, and detect its Tyndall phenomenon.
[0143] 1. material (with embodiment 1)
[0144] 2. Method
[0145] Propylene glycol with a concentration ≥ 50% by volume is selected, and ginsenoside-Rd is added and dissolved at differ...
Embodiment 3
[0163] Example 3. Effect of Ginsenoside-Rd on Inflammation
[0164] In this experiment, carrageenan was used as an acute inflammation inducer, and it was injected subcutaneously in the foot of mice and rats to induce rat and mouse foot swelling models to observe the effect of ginsenoside-Rd on inflammation.
[0165] 1. Animals
[0166] 120 Kunming mice, half male and half female, clean grade, weighing 18-22 g, were purchased from the Experimental Animal Center of Lanzhou University, license number: SCXK (Gan) 20050007; 96 Wistar rats, half male and half male, SPF grade, Body weight 180-220g, purchased from the Scientific Research and Experiment Center of Gansu College of Traditional Chinese Medicine, license number: SCXK (Gan) 20040006.
[0167] 2. Drugs
[0168] 2.1 Ginsenoside-Rd: provided by Guangdong Tahoe Bio-Pharmaceutical Co., Ltd., product batch number: 980303, dissolved in 55% propylene glycol to prepare a solution of corresponding concentration one day before use. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com