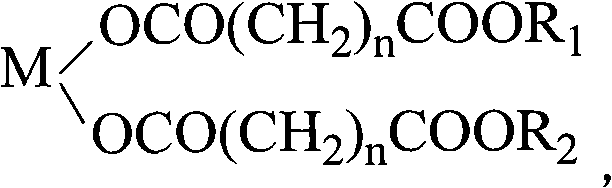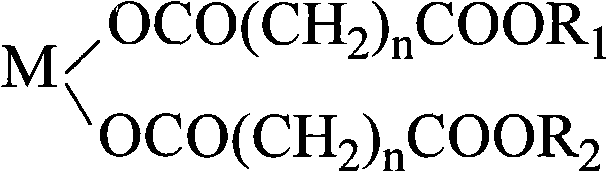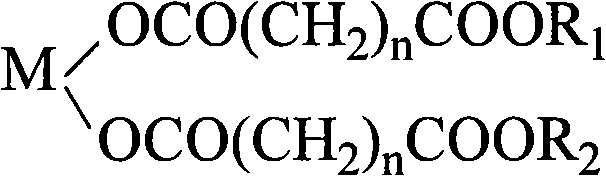PVC heat stabilizer of metal carboxylate of pentaerythritol ester and preparation method thereof
A technology of metal carboxylate and pentaerythritol ester, which is applied in the preparation of dipentaerythritol esterification, carboxylate and pentaerythritol, can solve the problem of complex components, and achieve simple post-treatment, small residue and easy processing Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Melt 66g of glutaric acid at 100-110°C, add 18g of calcium oxide, stir for 0.5 hours, mix well, add 5.7g of 30% hydrogen peroxide, and keep warm at 100-110°C for 1 hour. Add 109g of pentaerythritol and 51g of dipentaerythritol, stir evenly and heat up to 185±5°C to melt, then add 0.16g of p-toluenesulfonic acid and 8g of xylene and react at 185±5°C for 1 to 2 hours, then cool, crush and grind to obtain pentaerythritol Ester calcium salt PVC heat stabilizer (referred to as calcium salt I).
Embodiment 2
[0028] Melt 97.4g of adipic acid at 160-170°C, add 28g of zinc oxide, stir for 0.5 hour, mix well, add 12.6g of 30% hydrogen peroxide, and keep warm at 160-170°C for 2 hours. Add 77g of pentaerythritol and 71g of dipentaerythritol, stir evenly and heat up to 190±5°C to melt, add 0.29g of p-toluenesulfonic acid and 22g of xylene and react at 190±5°C for 1 to 2 hours, then cool, crush and grind to obtain pentaerythritol ester Zinc salt PVC heat stabilizer (referred to as zinc salt II).
Embodiment 3
[0030] Melt 133g of pimelic acid at 110-120°C, add 13.4g of magnesium oxide, stir for 0.5 hours, mix well, add 24g of 30% hydrogen peroxide, and keep warm at 110-120°C for 1.5 hours. Add 128g of pentaerythritol and 79g of dipentaerythritol, stir evenly, heat up to 190±5°C to melt, add 1.0g of phosphotungstic acid and 51.7g of xylene and react at 190±5°C for 1 to 2 hours, then cool, crush and grind to obtain pentaerythritol ester Magnesium salt PVC heat stabilizer (referred to as magnesium salt III).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com