I-type crystallization of N-n-propyl-3-(4-methylphenyl)-4-(4-mesylphenyl)-2,5-dihydropyrrole-2-ketone and manufacturing method thereof
A technology of methanesulfonyl phenyl and methyl phenyl, which is applied in the field of crystal form of antibacterial drugs, can solve the problems of fine crystallization, difficult filtration, poor fluidity, etc. The effect of crystal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
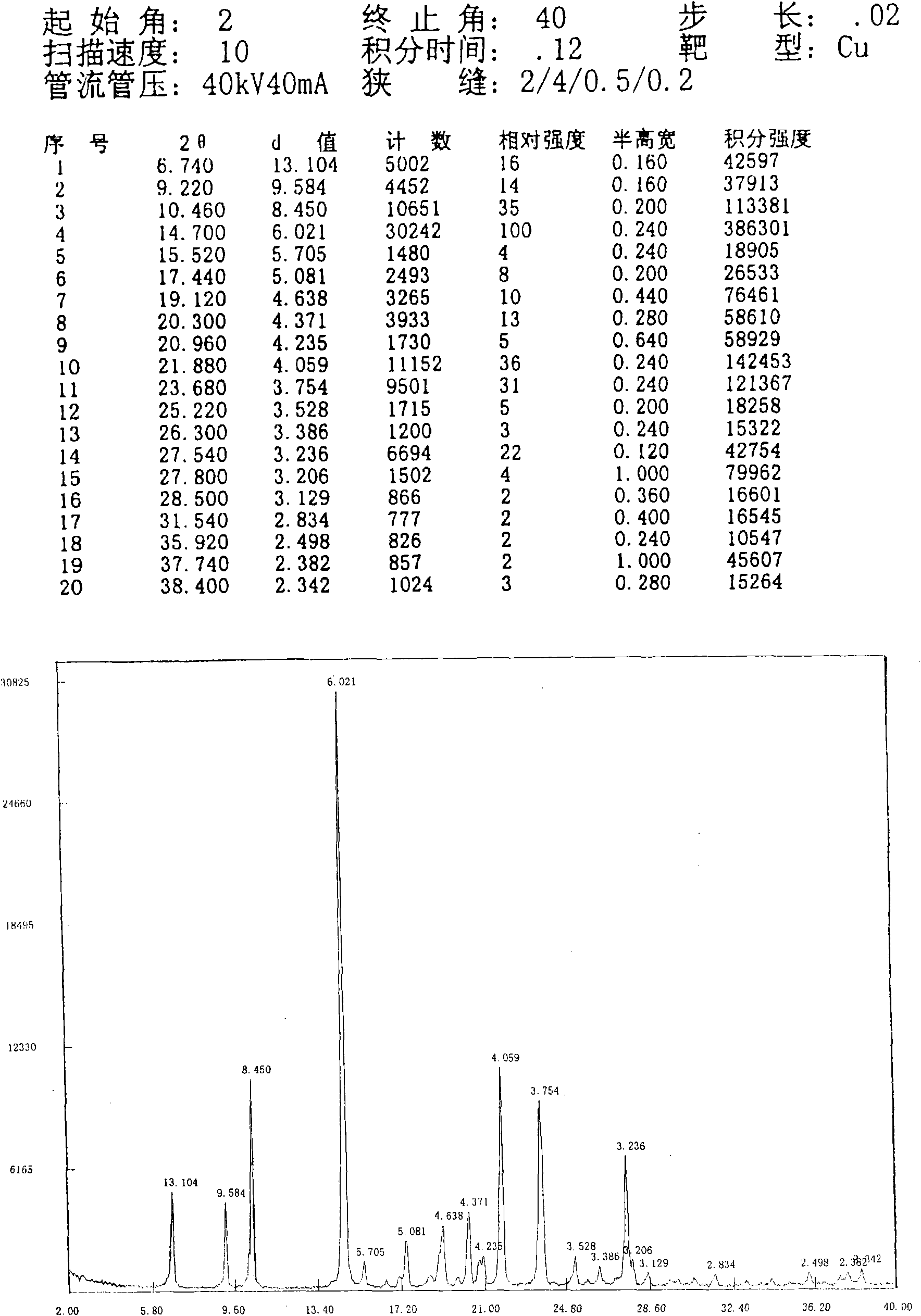
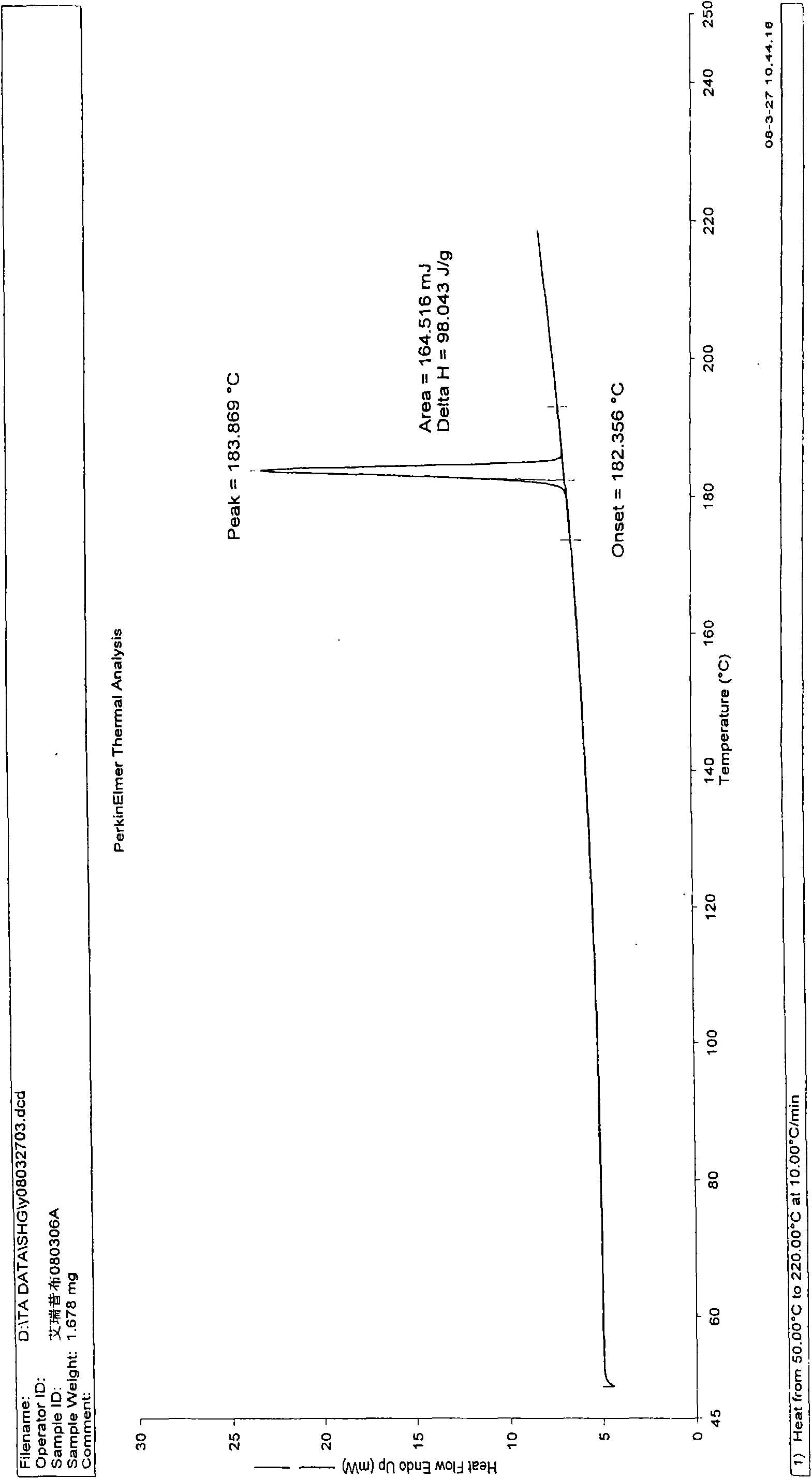
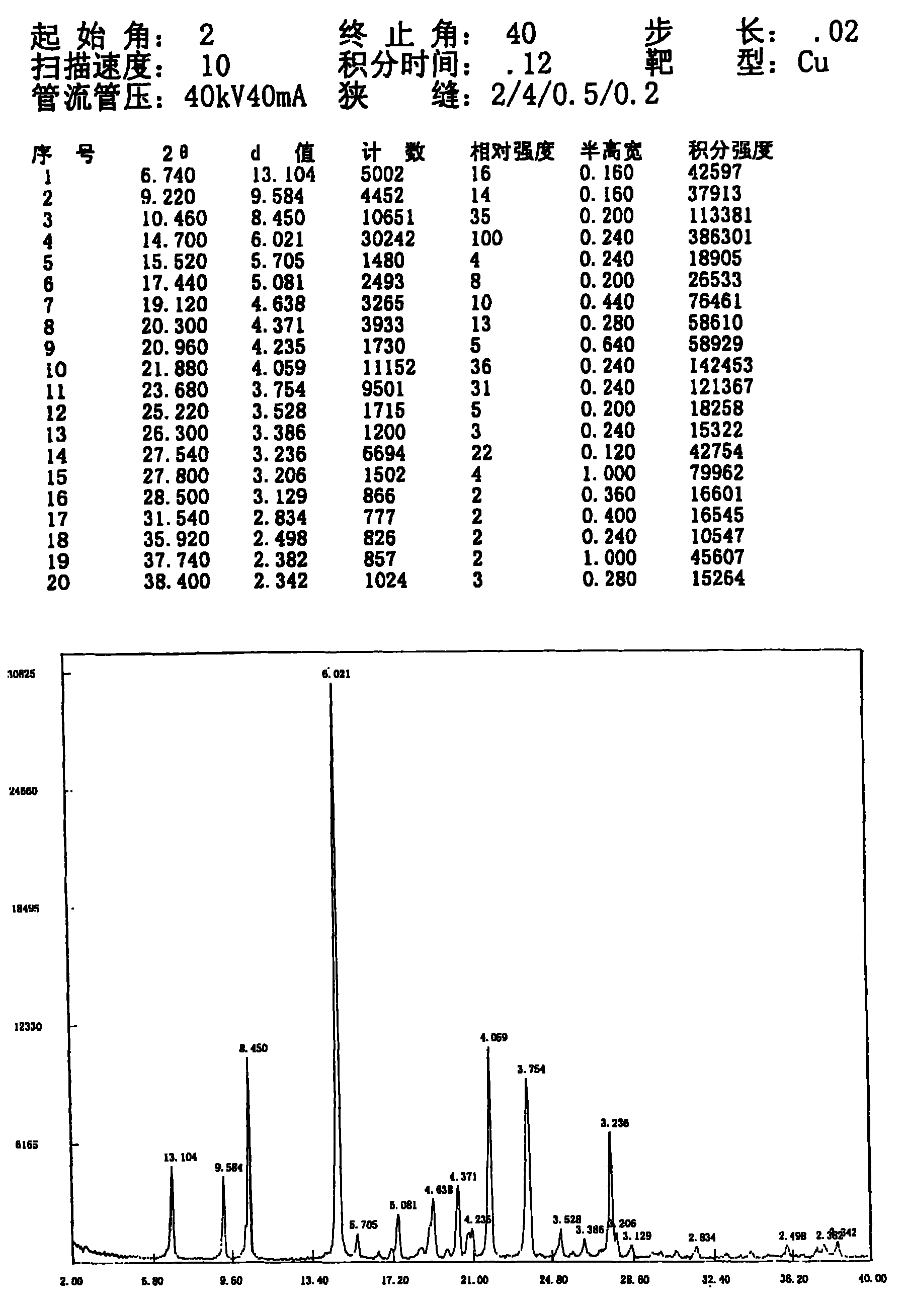
[0036] N-n-propyl-3-(4-methylphenyl)-4-(4-methylsulfonylphenyl)-2,5-dihydropyrrol-2-one 10.0g (according to patent CN1318541A disclosed method) was added into ethanol (150ml), heated to reflux to dissolve, slowly cooled to crystallize, and continued to crystallize to room temperature, and the precipitated crystals were collected by filtration. The obtained crystals were dried under reduced pressure at room temperature overnight to obtain 8.86 g of crystals with a yield of 88.6%. The residual ethanol solvent in the obtained sample is 100ppm, and the melting point measured by capillary method is 181.0~182.0°C; the X-ray diffraction spectrum of the crystalline sample is shown in figure 1 , is a type I crystal. See DSC Spectrum figure 2 , with a sharp melting endothermic peak of 183.87°C. The sample was dried under reduced pressure at 80° C. for 4 hours. The melting point of the dried sample was measured by capillary method to be 181.5-183.0° C., and the X-ray diffraction pa...
Embodiment 2
[0038] Add 10.0 g of N-n-propyl-3-(4-methylphenyl)-4-(4-methylsulfonylphenyl)-2,5-dihydropyrrol-2-one into methanol (150ml), Heat to reflux to dissolve, slowly cool to 35°C to start crystallization, continue to crystallize to room temperature, and collect the precipitated crystals by filtration. The obtained crystals were dried under reduced pressure at room temperature overnight to obtain 8.3 g of crystals, with a yield of 83%. Gained sample methanol solvent residual 200ppm, capillary method measures melting point 181.0~183.0 ℃; The characteristic peak of the X-ray diffraction spectrum of this crystalline sample is the same as figure 1 , is a type I crystal. The DSC spectrum has a sharp melting endothermic peak at 184.0°C. Then the sample was dried under reduced pressure at 80° C. for 4 hours. The melting point of the dried sample was measured by capillary method to be 181.5-183.0° C., and the X-ray diffraction spectrum was type I crystal. The DSC spectrum has a sharp melt...
Embodiment 3
[0040] Add 10.0 g of N-n-propyl-3-(4-methylphenyl)-4-(4-methylsulfonylphenyl)-2,5-dihydropyrrol-2-one to 50% ethanol (200ml) , heated to reflux to dissolve, slowly cooled to crystallize, crystals began to precipitate at 65°C, a large amount of crystals precipitated at 60°C, continued to crystallize to room temperature, and the precipitated crystals were collected by filtration. The obtained crystals were dried under reduced pressure at 60° C. overnight to obtain 9.1 g of crystals with a yield of 91%. The resulting sample has 500ppm ethanol solvent residue, contains 0.45% water, and has a melting point of 181.0-182.0°C measured by capillary method; the X-ray diffraction spectrum of the crystal sample is type I crystal. The DSC spectrum has a sharp melting endothermic peak at 184.42°C. Then the sample was vacuum-dried at 80°C for 4 hours, the moisture content of the dried sample was 0.20%, the melting point was 181.0-182.5°C measured by capillary method, and the X-ray diffracti...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com