Phosphor
A technology of phosphors and metal elements, applied in the field of phosphors, can solve problems such as difficulty in reducing luminous brightness, and achieve the effect of reducing luminous brightness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0056] Next, the present invention will be described in detail through examples, but the present invention is not limited to these examples.
[0057] The measurement of the luminous brightness is carried out as follows. The phosphor is placed in a vacuum chamber and kept at 6.7Pa (5×10 -2 In a vacuum below Torr), vacuum ultraviolet rays were irradiated using an excimer laser 146 nm light source (Usio Denki Co., Ltd. H0012 type) or an excimer laser 172 nm light source (Usio Denki Co., Ltd. H0016 type).
[0058] The content of the halogen element in the phosphor was obtained by the following method.
[0059] That is, put 1 g of a weighed phosphor powder sample into a distillation flask together with pyrophosphoric acid, heat and dissolve the phosphor powder, and then introduce water vapor into the flask (the flask is kept at 145° C.) to fully extract the halogen to On the water vapor side, the water vapor is cooled to obtain a brine extraction solution (about 500 ml of the obtain...
Embodiment 1
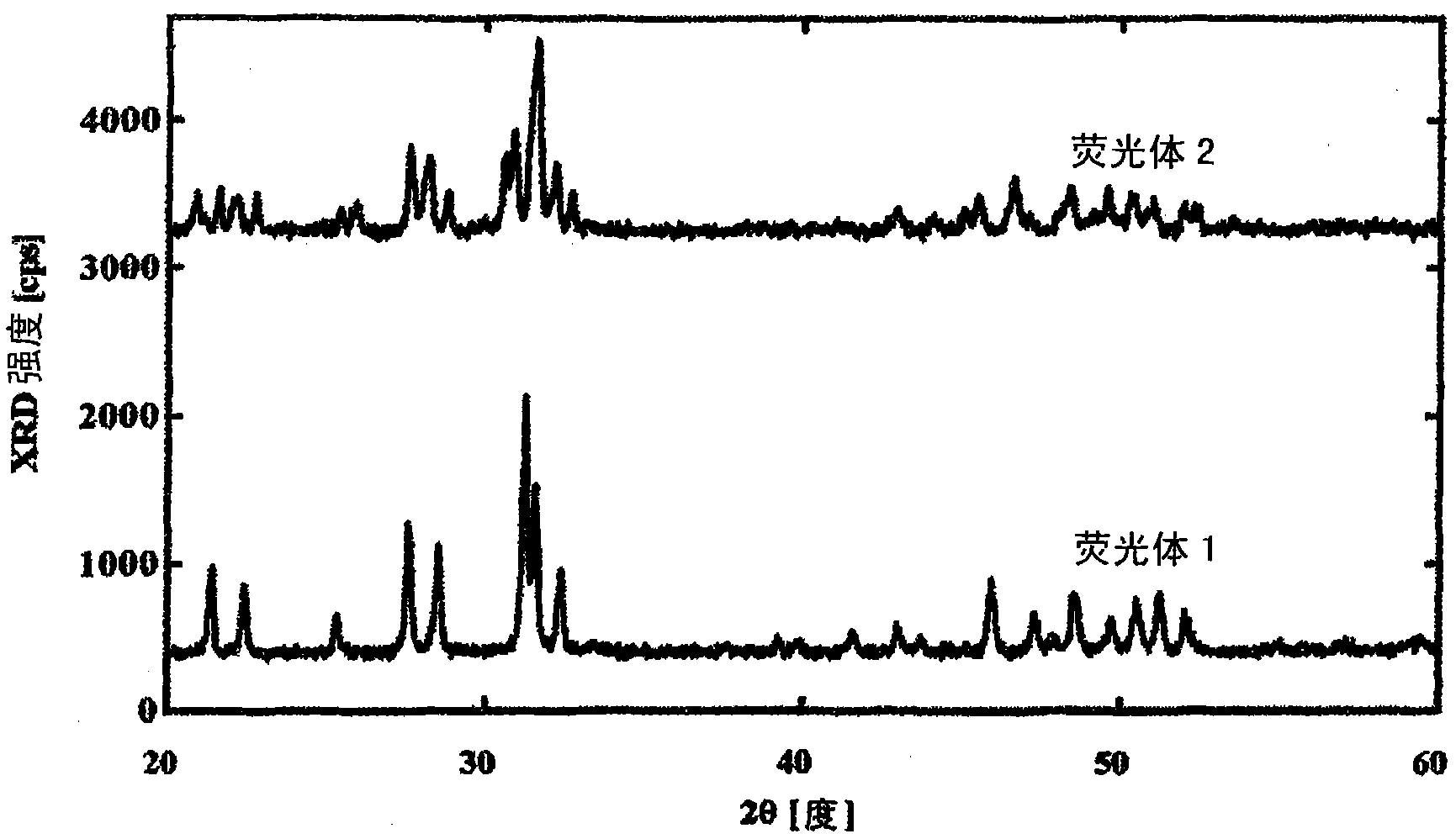
[0068] Barium carbonate (manufactured by Kanto Chemical Co., Ltd.: purity 99.99%), gadolinium oxide (manufactured by Shin-Etsu Chemical Co., Ltd.: purity 99.99%), terbium fluoride (manufactured by Kanto Chemical Co., Ltd.: purity 99.99%), and silica (and Mitsujun Pharmaceutical Co., Ltd.: purity 99.99%), weighed and mixed in a molar ratio of Ba:Gd:Tb:Si of 1:3.4:0.6:3, and contained 2% by volume of H 2 N 2 It was kept at 1000° C. for 3 hours and sintered, and then slowly cooled to room temperature to obtain phosphor 2 . figure 1 The X-ray diffraction pattern of phosphor 2 is shown. according to figure 1 It is found that the X-ray diffraction pattern of the phosphor 2 is different from that of the phosphor 1 . In addition, the content of fluorine (F) in the phosphor 2 was investigated and found to be 25000 ppm, and the molar ratio of Ba:(Gd+Tb):Si:F of the phosphor was 1:4:3:1.4.
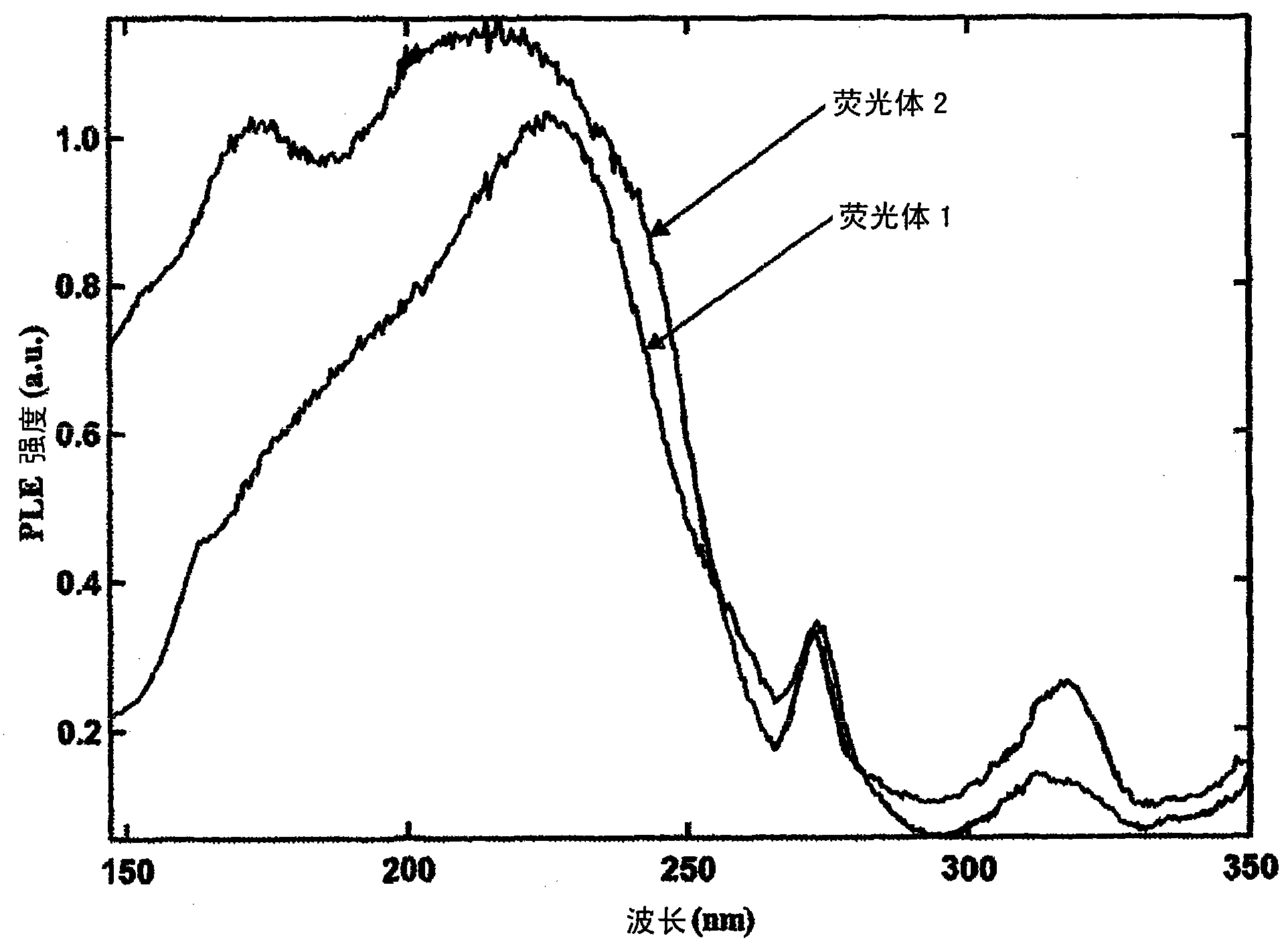
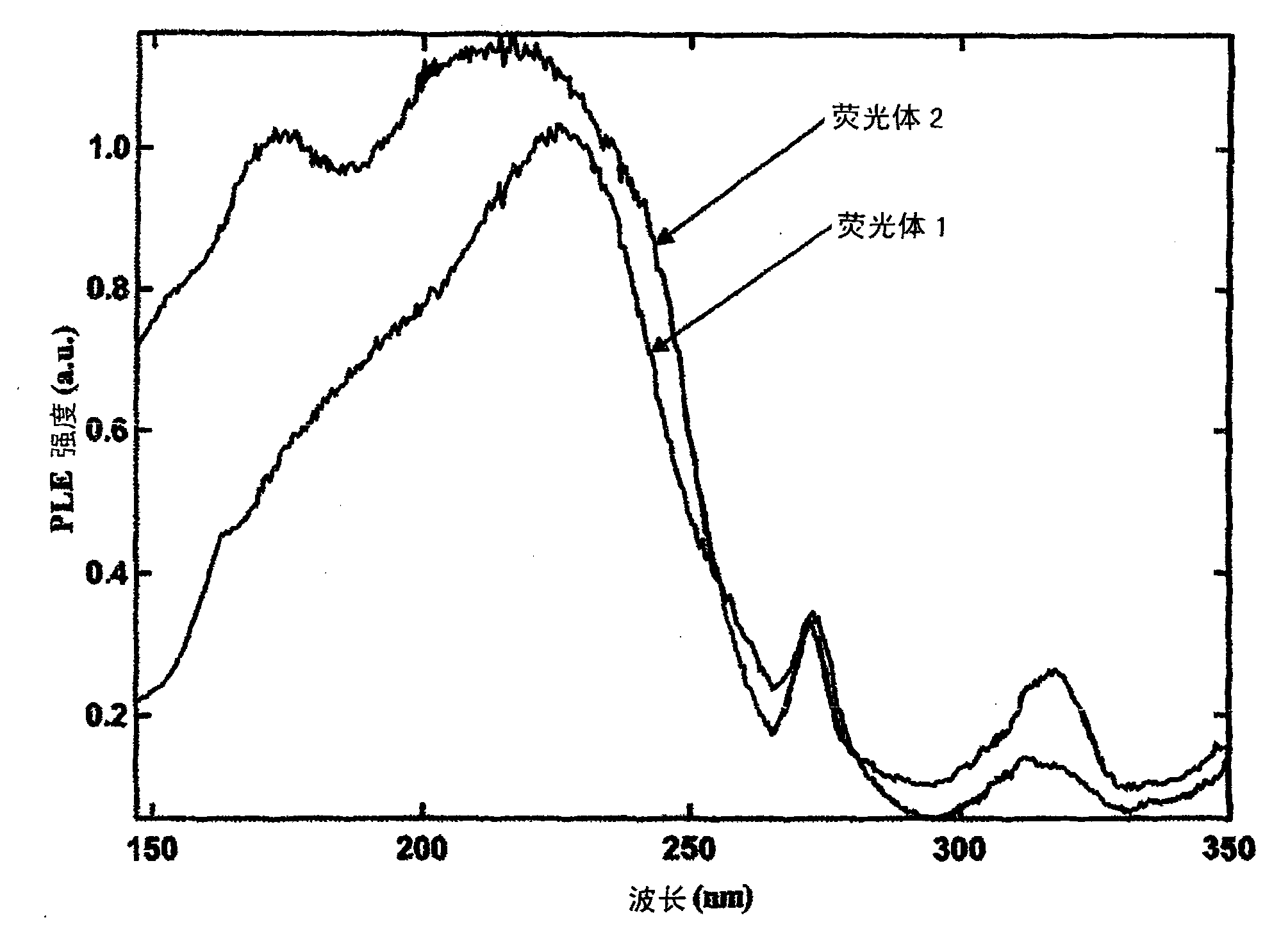
[0069] Phosphor 2 at 6.7Pa (5×10 -2 After irradiating it with vacuum ultraviolet rays using ...
Embodiment 2
[0073] Barium carbonate (manufactured by Kanto Chemical Co., Ltd.: purity 99.99%), gadolinium oxide (manufactured by Shin-Etsu Chemical Co., Ltd.: purity 99.99%), gadolinium fluoride (manufactured by Kanto Chemical Co., Ltd.: purity 99.99%) and terbium oxide (manufactured by Shin-Etsu Chemical Co., Ltd.) were used. Kogyo Co., Ltd.: purity 99.99%) and silicon dioxide (Wako Junpharmaceutical Co., Ltd.: purity 99.99%), barium carbonate (BaCO 3 ): Gadolinium oxide (Gd 2 o 3 ): Gadolinium Fluoride (GdF 3 ): Terbium oxide (Tb 4 o 7 ): silicon dioxide (SiO 2 ) in a molar ratio of 1:1.4:0.6:0.15:3 after weighing and mixing, in the presence of 2% by volume H 2 N 2 It was kept at 1000° C. for 3 hours and sintered in the environment, and then slowly cooled to room temperature to obtain phosphor 3 . In addition, the content of fluorine (F) in the phosphor 3 was investigated and found to be 25000 ppm, and the molar ratio of Ba:(Gd+TB):Si:F in the phosphor 3 was 1:4:3:1.4.
[0074] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com