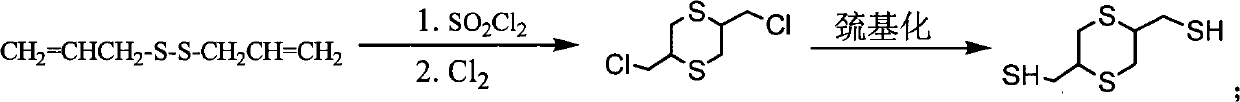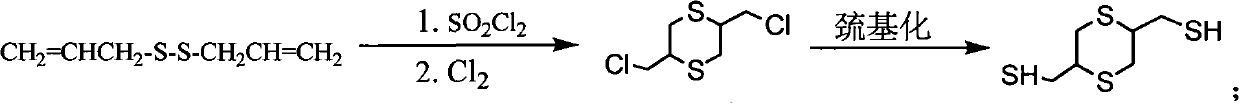Method for preparing 2,5-dimercapto-methyl-1,4-dithiane
A technology of dimercaptomethyl and dithiane, which is applied in the field of optical materials, can solve the problems of complicated operation process and high cost, and achieve the effect of simple method, light weight and high refractive index
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0028] (1) Put a 250mL three-neck flask on a magnetic stirrer with a heating device, install a thermometer and a condenser tube, and add Na 2 S.9H 2 O 63.4g (0.264mol), water 130mL, start stirring and heating to 50°C until Na 2 S.9H 2 After O is completely dissolved, add 8.45g (0.264mol) of sulfur powder and react at this temperature for 1h to obtain an aqueous sodium disulfide solution, add 4000.5g of phase transfer catalyst polyethylene glycol, then cool to 20°C and then add 3-chloropropene dropwise 39.2mL, after the dropwise addition was completed, the water bath was heated to 50°C to react for 3h. The organic layer was separated and dried, and then distilled under reduced pressure to obtain 28.8 g of colorless diallyl disulfide.
[0029] (2) Add 25g (0.17mol) of diallyl disulfide and 50mL of dichloroethane to the three-necked flask, and add 25g (0.185mol) of sulfuryl chloride dropwise in an ice-water bath to control the temperature at 3-10°C. Stir at 20°C for 3 hours. ...
example 2
[0033] Step (1) in the method in the example 1 obtains 25g (0.17mol) of diallyl disulfide, solvent methylene chloride 50mL, ice-water bath control temperature 3-10 ℃ feeds chlorine gas 13.3g (0.187mol), completes After stirring at 20°C for 3 hours, an orange liquid was obtained after the reaction was completed, and then the orange liquid was poured into a saturated sodium bicarbonate solution to wash fully, and the pH was adjusted to neutral, then the organic layer was separated and dried, and the solvent was distilled off and then distilled under reduced pressure to obtain Yellow fraction 2,5-bis(chloromethyl)-1,4-dithiane 15.3g (156°C / 8mmHg).
[0034] Add 15g (0.07mol) of 2,5-bis(chloromethyl)-1,4-dithiane and 50mL of absolute ethanol as a solvent into a three-necked flask, then add 10.6g (0.14mol) of thiourea, and heat to reflux for 1h After the reaction was completed, it was filtered and washed several times with absolute ethanol to obtain 18.1 g of white solid isothiouron...
example 3
[0037] The method in example 1 obtains 25g g (0.17mol) of diallyl disulfide, solvent dichloroethane 50mL, ice-water bath control temperature 3-10 ℃, dropwise add sulfuryl chloride 25.2g (0.187mol), dropwise Stir at 20°C for 3 hours. After the reaction, an orange liquid is obtained. Then, the orange liquid is poured into a saturated sodium bicarbonate solution and washed fully to adjust the pH to neutral. The organic layer is separated and dried. The solvent is evaporated and then distilled under reduced pressure to obtain a yellow color. Fraction 2,5-bis(chloromethyl)-1,4-dithiane 156°C / 8mmHg 21.8g.
[0038] Add 21.4g (0.099mol) of 2,5-bis(chloromethyl)-1,4-dithiane and 50mL of water into a three-necked flask, then add 15g (0.197mol) of thiourea, and protect the temperature at 90°C with nitrogen gas React for 3 hours. After the reaction is completed, add 39.4g of 20% NaOH solution dropwise, and react at 90°C for 3 hours. After the reaction, adjust the pH to about 3 with dilute...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com