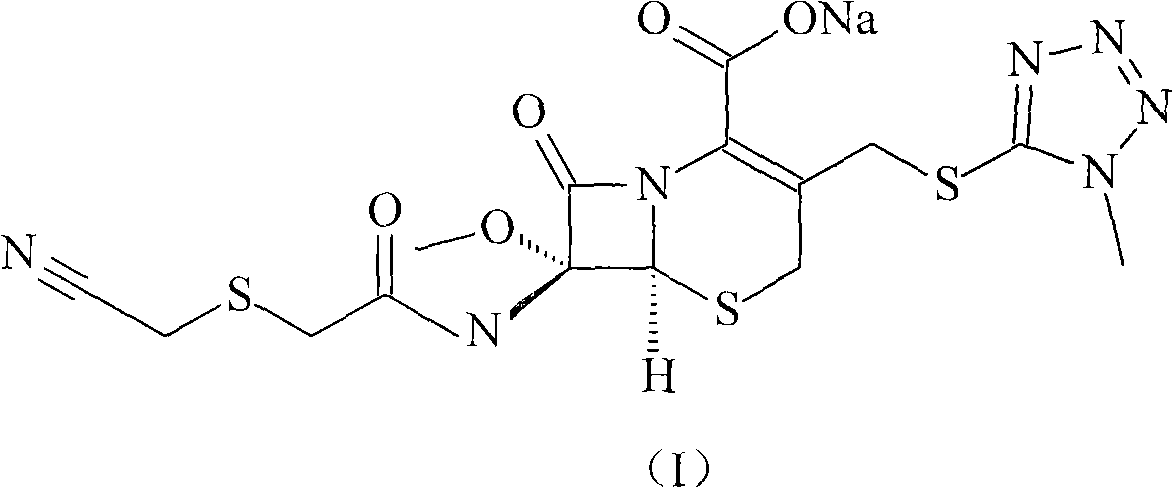
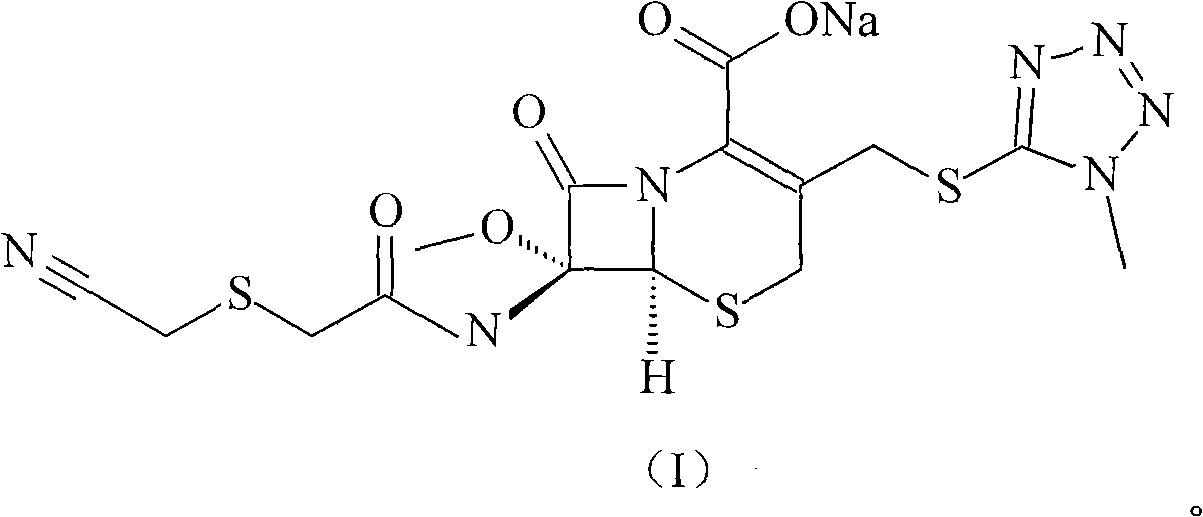
High-purified cefmetazole sodium compound
A technology of cefmetazole sodium and cefmetazole, applied in the field of medicine, can solve problems such as poor purity, adverse reactions, poor color, etc., and achieve the effects of improving purity and content, optimizing product quality, and ensuring safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] The refining of embodiment 1 cefmetazole sodium
[0032] (1) Dissolve 200g of cefmetazole sodium in 2000ml of water, add 0.5mol / L hydrochloric acid solution to a pH value of 2.2, precipitate insoluble matter, stir and react for 60 minutes, filter, and dry under reduced pressure at 40°C to obtain cefmetazole 180.8 g;
[0033] (2) Dissolve 180.8g cefmetazole in 2000ml of acetonitrile and ethyl acetate solution with a volume ratio of 1:2, add D1300 type macroporous adsorption resin, stir and adsorb at room temperature for 20 minutes, and use acetonitrile with a volume ratio of 1:2 Eluted and purified with ethyl acetate solution, collected the eluate, added 3.01 g of activated carbon, stirred and adsorbed at room temperature for 30 minutes, filtered for decarburization, and collected the filtrate;
[0034] (3) Add dropwise 4% sodium hydroxide solution to pH 8.0 in the filtrate, separate out insolubles, stir and react at room temperature for 60 minutes, filter, and dry und...
Embodiment 2
[0035] The refining of embodiment 2 cefmetazole sodium
[0036] (1) Dissolve 200g of cefmetazole sodium in 2000ml of water, add 2mol / L sulfuric acid solution to a pH value of 2.6, precipitate insoluble matter, stir and react for 30 minutes, filter, and dry under reduced pressure at 50°C to obtain cefmetazole 182.6 g;
[0037] (2) Dissolve 182.6g of cefmetazole in 2000ml of methanol, add BS-55 type macroporous adsorption resin, stir and adsorb at room temperature for 30 minutes, elute and purify with methanol, collect the eluate, add 4.02g of activated carbon, and stir at room temperature Adsorb for 20 minutes, filter for decarburization, and collect the filtrate;
[0038] (3) Add dropwise 10% sodium carbonate solution to the pH value of 7.0 in the filtrate, precipitate insoluble matter, stir and react at room temperature for 30 minutes, filter, and dry under reduced pressure at 50°C to obtain 181.8g of cefmetazole sodium, with a yield of 90.9%, HPLC The purity is 99.6%.
Embodiment 3
[0039] The refining of embodiment 3 cefmetazole sodium
[0040] (1) Dissolve 200g of cefmetazole sodium in 23000ml of water, add 1mol / L formic acid solution until the pH value is 2.7, precipitate insoluble matter, stir and react for 40 minutes, filter, and dry under reduced pressure at 50°C to obtain 181.2g of cefmetazole ;
[0041] (2) Dissolve 181.2g cefmetazole in 2000ml isopropanol, add D1300 type macroporous adsorption resin, stir and adsorb at room temperature for 30 minutes, elute and purify with isopropanol, collect eluent, add 2.08g of gac, Stir and adsorb at room temperature for 30 minutes, filter for decarburization, and collect the filtrate;
[0042] (3) Add 2% sodium acetate solution dropwise to pH 7.5 in the filtrate, precipitate insoluble matter, stir and react at room temperature for 45 minutes, filter, and dry under reduced pressure at 40°C to obtain 183.4g of cefmetazole sodium, yield 91.7%, HPLC The purity is 99.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com