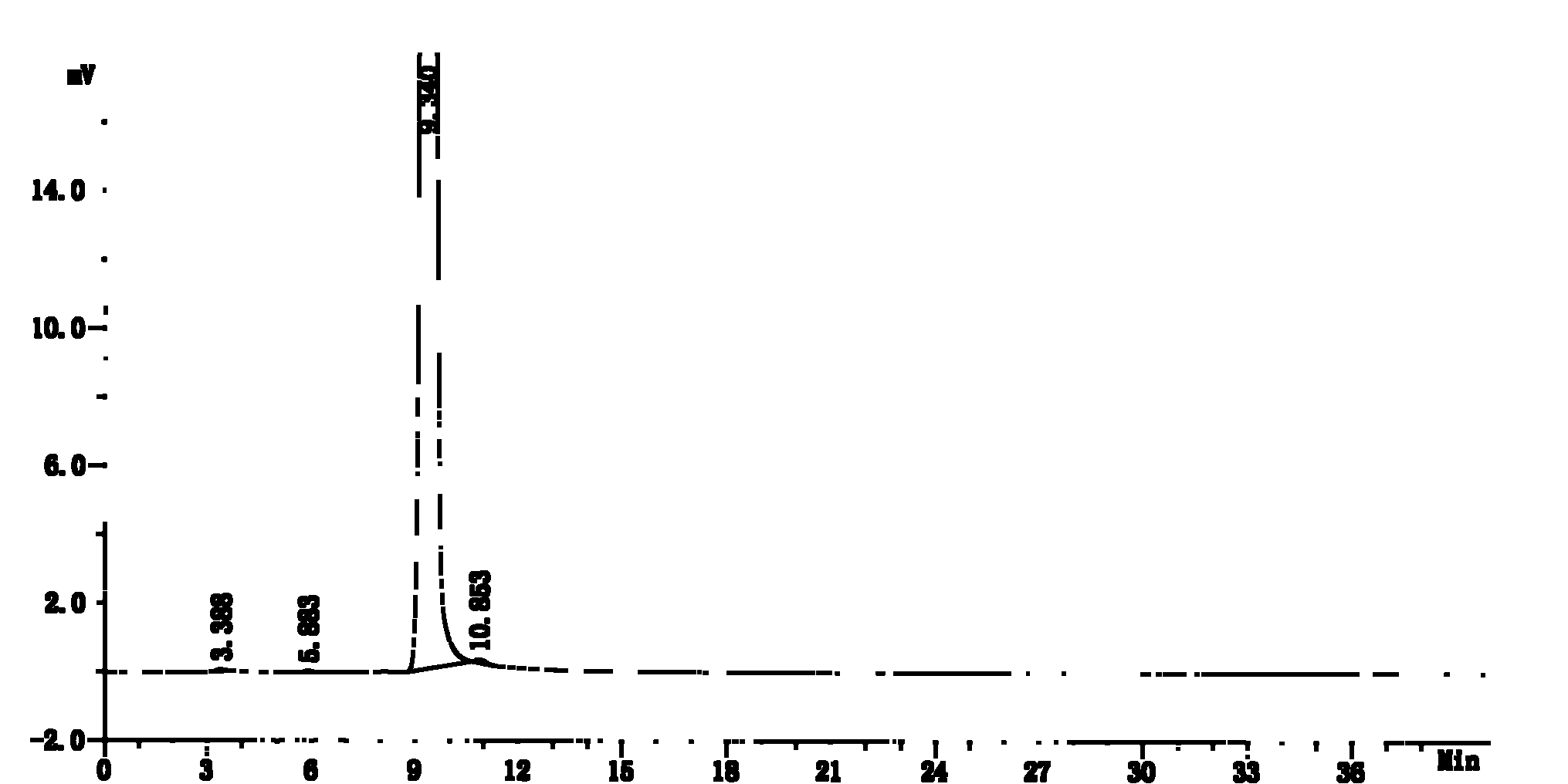
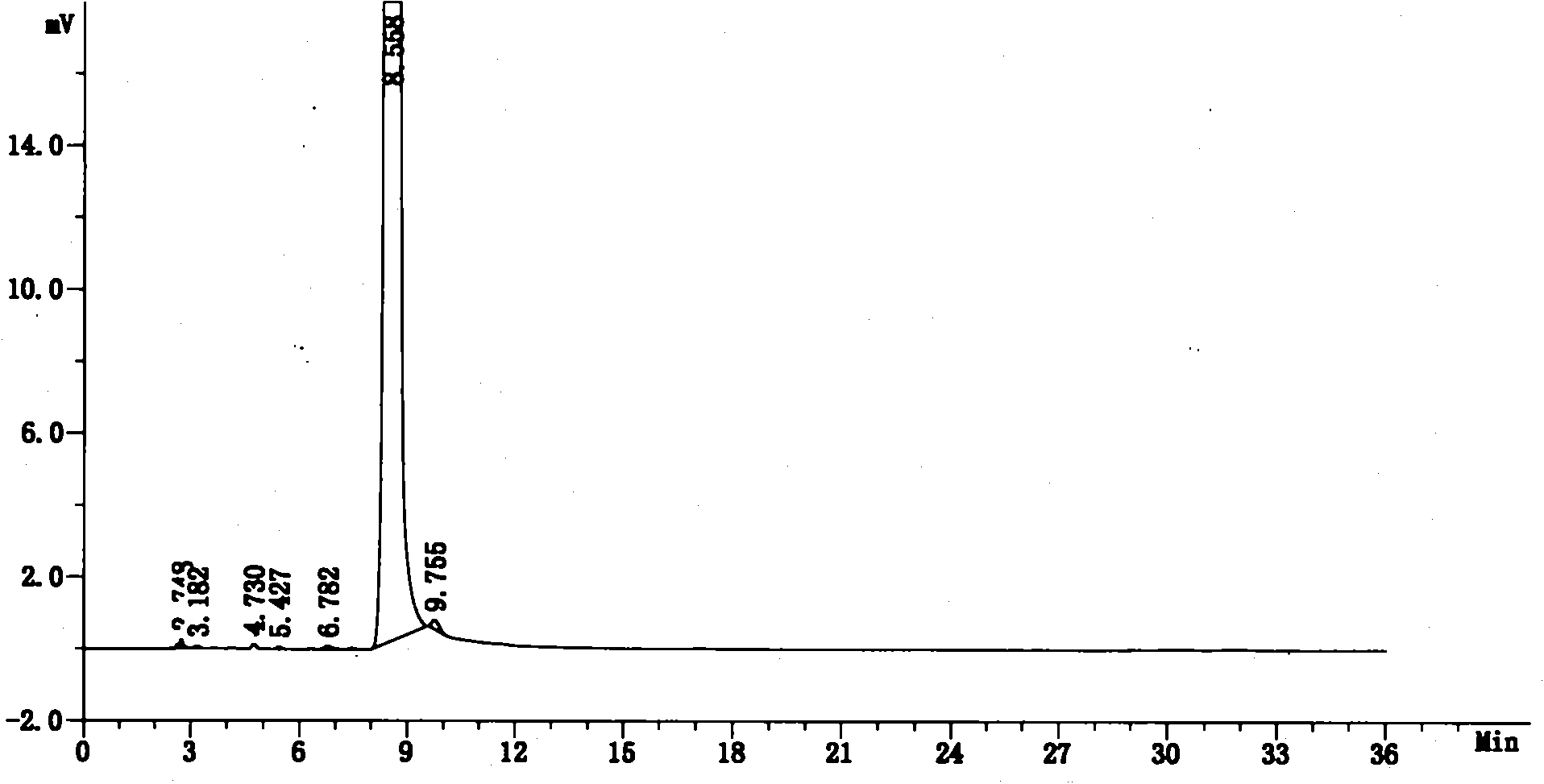
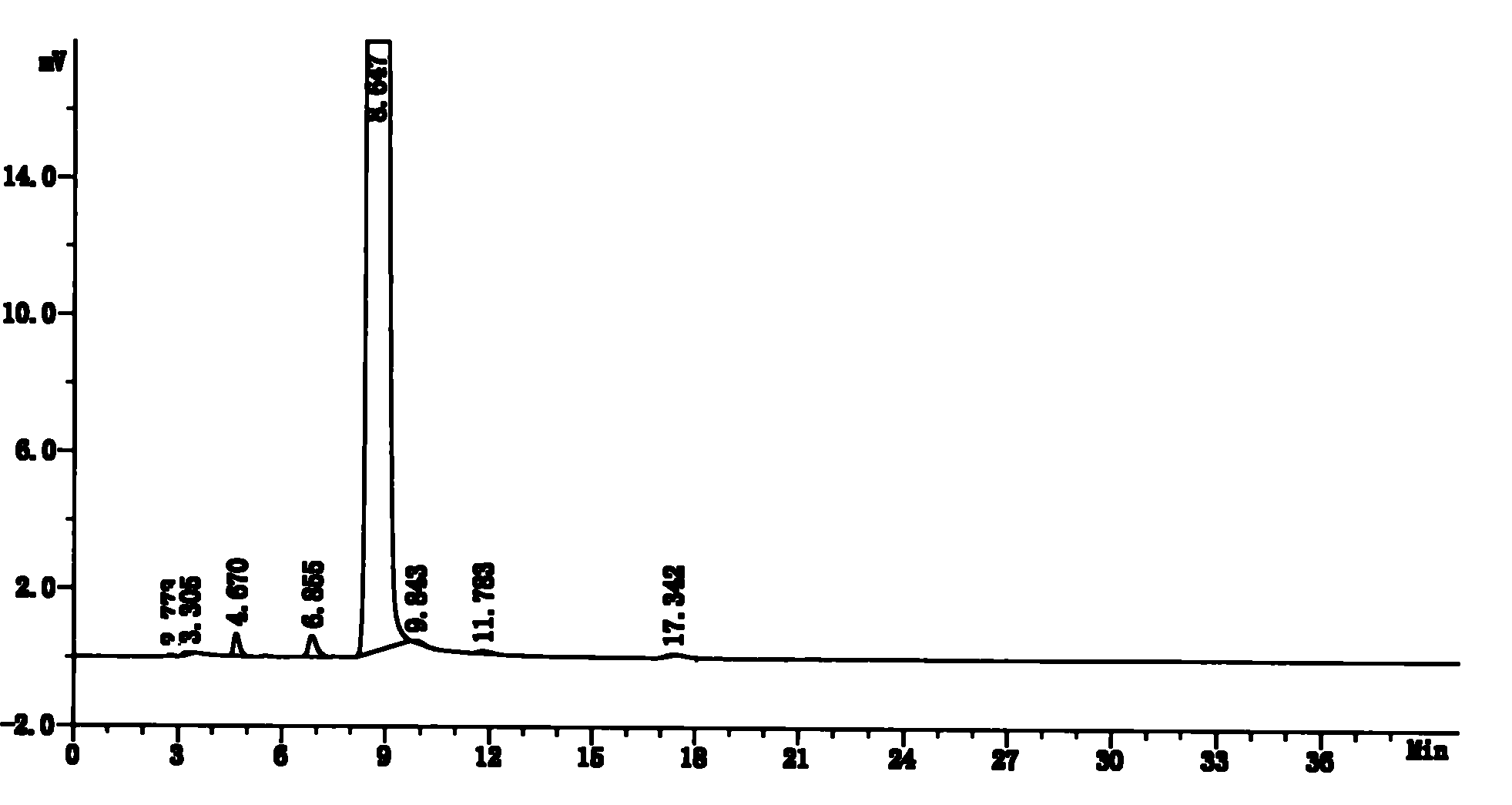
Stable lenalidomide oral solid preparation
A technology of lenalidomide and solid preparations, which is applied in the field of pharmaceutical preparations, can solve the problems of changing appearance and color, affecting the stability of preparations, and increasing the impurity content of solid oral preparations, achieving improved stability, low impurity content, and high solubility Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083] 5mg Lenalidomide Capsules
[0084] Premix Powder Prescription
[0085] Lactose 3000g
[0086] Microcrystalline cellulose 2000g
[0087] Total 5000g
[0088] Attachment: the preparation of adhesive (10% povidone ethanol solution):
[0089] Povidone K30 200g
[0090] Add absolute ethanol to 2000ml
[0091] capsule prescription
[0092] Lenalidomide 50g
[0093] Premixed accessories 800g
[0094] Croscarmellose Sodium 100g
[0095] 10% povidone ethanol solution 350ml
[0097] Fill 10000 capsules
[0098] Preparation:
[0099] (1) Preparation of adhesive Weigh 100g of povidone K30, put it in a 1000ml beaker, add an appropriate amount of absolute ethanol, stir to dissolve, add absolute ethanol to 1000ml, stir well, and set aside.
[0100] (2) Take lactose and microcrystalline cellulose, grind them separately, pass through a 100-mesh sieve, and mix evenly according to the prescription of the premixed auxiliary material to obtain th...
Embodiment 2
[0110] 10mg Lenalidomide Capsules
[0111] Premix powder prescription is the same as embodiment 1
[0112] capsule prescription
[0113] Lenalidomide 100g
[0114] Premixed accessories 750g
[0115] Croscarmellose Sodium 100g
[0116] 10% povidone ethanol solution 350ml
[0118] Fill 10000 capsules
[0119] Preparation method: with embodiment 1.
Embodiment 3
[0121] 25mg Lenalidomide Capsules
[0122] Premix powder prescription is the same as embodiment 1
[0123] capsule prescription
[0124] Lenalidomide 250g
[0125] Premixed accessories 600g
[0126] Croscarmellose Sodium 100g
[0127] 10% povidone ethanol solution 350ml
[0129] Fill 10000 capsules
[0130] Preparation method: with embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com