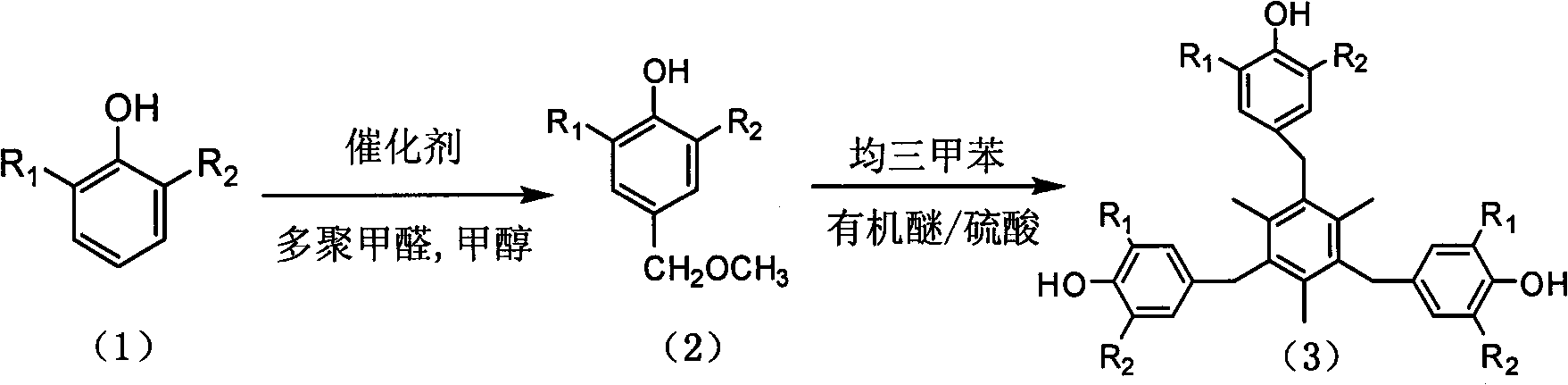
Synthesis method of polysubstituted hindered phenol antioxygen
A technology of hindered phenol antioxidant and synthesis method, which is applied in the direction of chemical instruments and methods, preparation of organic compounds, production of bulk chemicals, etc., and can solve problems such as high yield, small amount of acid used, and impossibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Add 49.6g 2,6-di-tert-butylphenol, 200mL methanol, 12.1g paraformaldehyde, 1.5ml diethylamine, 3.0mL tetramethylethylenediamine into a 500mL autoclave, under the protection of nitrogen, at 70℃ , The reaction is 5h, and the reaction adopts a batch reactor;
[0066] After the reaction, the solvent and the catalyst diethylamine and tetramethylethylenediamine were directly distilled off under reduced pressure, and then cooled to room temperature to obtain 58.2g crude product of 3,5-di-tert-butyl-4-hydroxybenzyl methyl ether. , The content is 93.8%, the recovered solvent methanol, formaldehyde and catalyst are directly used in the next batch of reactions;
[0067] In a 250mL three-necked flask, at a temperature of 25℃, add 25mL dichloromethane, 2.8ml ether, 3ml 95% sulfuric acid, 1.2g mesitylene and 6.4g 3,5-di-tert-butyl-4-hydroxy under stirring. Benzyl methyl ether crude product, reaction temperature is 10℃, time is 0.5h, and then slowly flowed into 3.2g 3,5-di-tert-butyl-4-hy...
Embodiment 2
[0070] Add 49.6g 2,6-di-tert-butylphenol, 13.6g paraformaldehyde, 200mL methanol, 1.5ml dipropylamine, 3.0mL tetramethylethylenediamine into a 500mL autoclave, under the protection of nitrogen, at a temperature of 100℃ , The reaction is 4h, and the reaction adopts a continuous reactor;
[0071] After the reaction, part of the solvent methanol, formaldehyde, catalyst diethylamine and tetramethylethylenediamine were evaporated, then cooled to room temperature, crystallized and filtered to obtain crude 3,5-di-tert-butyl-4-hydroxybenzyl methyl ether It is 58.4g and the content is 93.2%. The recovered solvent methanol, formaldehyde and catalyst are directly used in the next batch of reactions;
[0072] In a 250mL three-necked flask at 15℃, add 25mL dichloromethane, 3.2ml tetrahydrofuran, 3ml 90% sulfuric acid, 1.2g mesitylene and 6.4g 3,5-di-tert-butyl-4-hydroxyl group under stirring. Benzyl methyl ether crude product, reaction temperature is 20℃, time is 1.5h, and then slowly flowed i...
Embodiment 3
[0075] Add 49.6g 2,6-di-tert-butylphenol, 14.4g paraformaldehyde, 200mL 5% aqueous methanol solution, 1.5ml dipropylamine, 3.0mL tetramethylethylenediamine in a 500mL autoclave, under nitrogen protection, React at 110°C for 3h, using batch reactor for reaction;
[0076] After the reaction, under reduced pressure, the solvent and the catalyst diethylamine and tetramethylethylenediamine were directly distilled off, and then cooled to room temperature to obtain the crude product of 3,5-di-tert-butyl-4-hydroxybenzyl methyl ether 58.4 g, the content is 93.8%, the recovered solvent methanol, formaldehyde and catalyst are directly used in the next batch of reactions;
[0077] In a 250mL three-necked flask at a temperature of 40°C, add 25mL of dichloromethane, 1.7ml of dioxane, 3ml of 80% sulfuric acid, 1.2g of mesitylene and 6.4g of 3,5-di-tert-butyl under stirring. The crude product of 4-hydroxybenzyl methyl ether, the reaction temperature is 25℃, the time is 2h, and then 3.2g of crude ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com