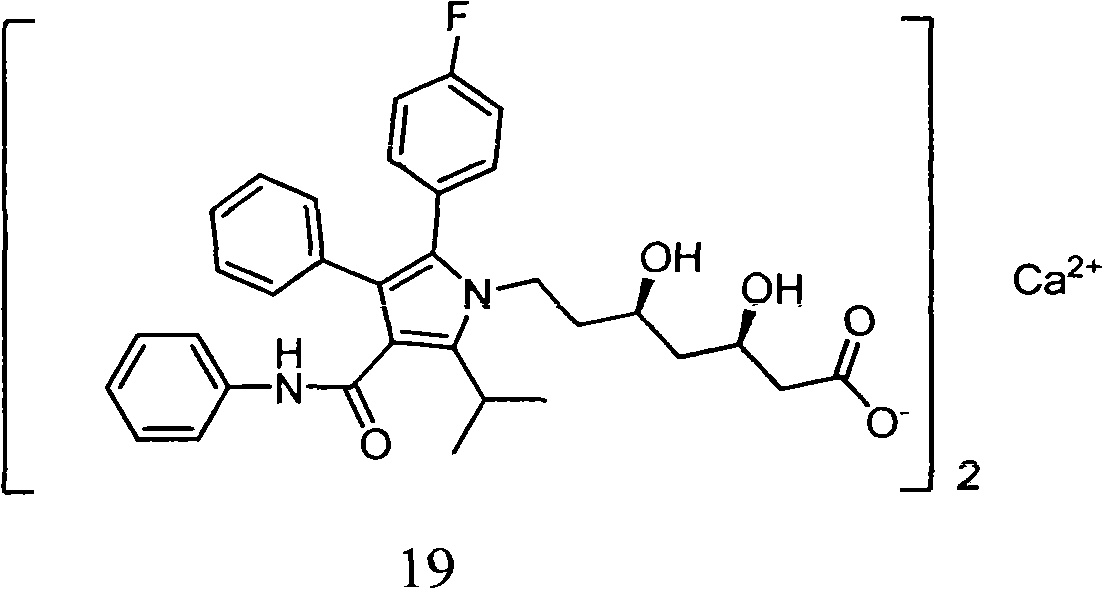
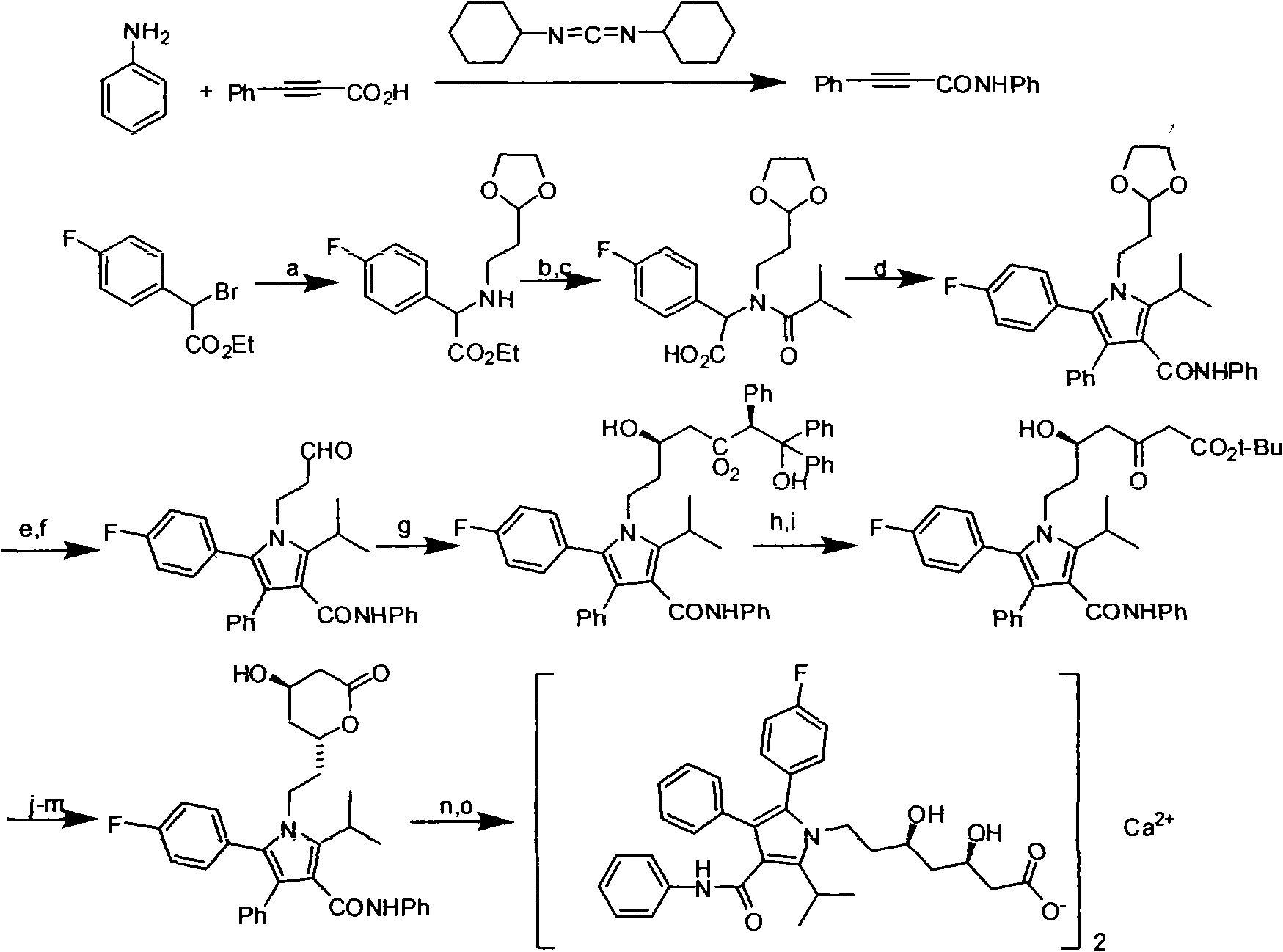
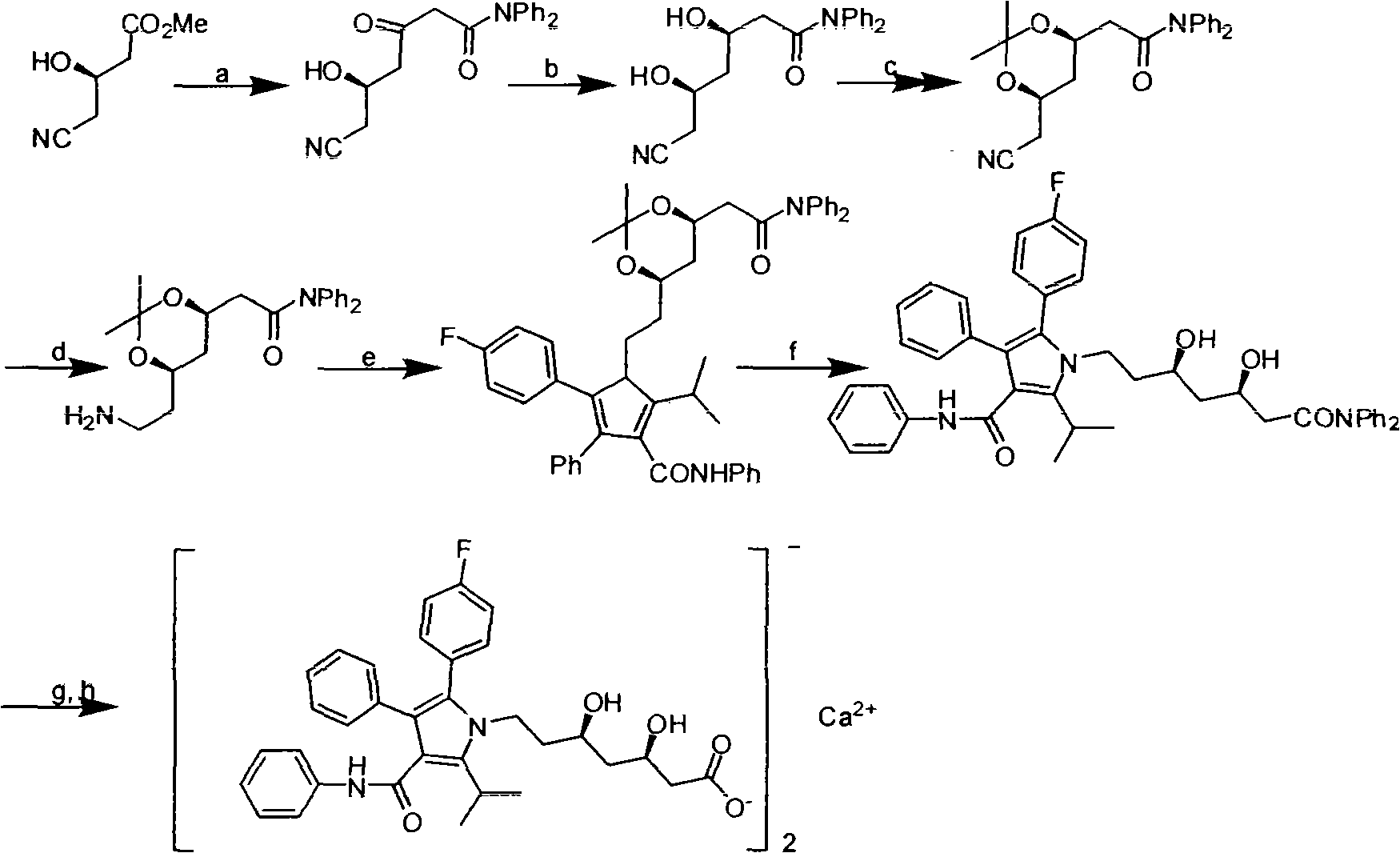
Preparation method of atorvastatin calcium
A technology of atorvastatin calcium and atorvastatin method, which is applied in the field of preparation of atorvastatin calcium, can solve the problems of expensive raw materials and high synthesis costs, and achieve the advantages of easy-to-obtain raw materials, high synthesis yield, and low-cost raw materials Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0033] In this example, in order to overcome the deficiencies of the prior art, atorvastatin calcium was synthesized using cheap and easily available natural products as raw materials. The preparation method of atorvastatin calcium is: synthesize the main ring fragment and the side chain fragment respectively, and then concatenate them to form the target product. The difference in chain fragments makes the process of connecting side chains and main rings and then synthesizing atorvastatin calcium different from the existing methods. The specific method is:
[0034] Synthesis of A main loop fragment
[0035] 1) Synthesis of methyl isobutyryl acetate (compound of formula 2) (see chemical equation 6),
[0036]
[0037] Chemical Equation 6
[0038] In a dry 250mL round bottom flask, add 0.20mol sodium hydride, add 150mL benzene, 16.6mL (0.12mol) dimethyl carbonate, and heat until the reaction liquid is refluxed. Add 50mL of benzene solution containing 5.727g (75mmol) of met...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com