Method for preparing honeycomb ceramic catalyst for catalytically ozonizing organic matters in water
A honeycomb ceramic, ozonated water technology, applied in metal/metal oxide/metal hydroxide catalysts, physical/chemical process catalysts, chemical instruments and methods, etc., can solve solid-liquid separation, catalyst recycling difficulties, metal Dissolution is serious and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
[0014] Specific implementation mode one: preparation of honeycomb ceramic catalyst
[0015] Soak the honeycomb ceramics in dilute hydrochloric acid solution, take it out and wash it with deionized water, then immerse it in the aluminum sol prepared from pseudo-boehmite for 2 minutes, blow off the excess sol in the pores after taking it out, dry at room temperature for 12 hours, and dry at 110°C 4 hours, and then calcined at 500° C. for 3 hours to obtain a once-coated honeycomb ceramic carrier. Repeat the above steps twice to obtain a three-time coated honeycomb ceramic carrier. Co(NO 3 ) 2 solution, soak the coated ceramic support in Co(NO 3 ) 2 Take it out after 1 hour in the solution, blow out the excess solution in the carrier channel, dry at 110°C for 3 hours, wash with deionized water, dry at 110°C for 3 hours, and calcined at 450°C for 3 hours.
[0016] After three coatings, the content of the alumina coating was 13.5wt%. The coated honeycomb ceramics were placed in...
specific Embodiment approach 2
[0019] Specific embodiment two: Catalytic ozone oxidation activity of honeycomb ceramic catalyst
[0020] In a 6L columnar bubbling semi-continuous reactor, 2,4-dichlorophenoxyacetic acid was used as the target pollutant to evaluate the catalytic ozonation activity of the honeycomb ceramic catalyst of the present invention. The reaction temperature is 25° C., pH=4-9, the gas flow rate of the ozone-oxygen mixed gas is 0.5 L / min, and the ozone concentration in the gas is 30 mg / L.
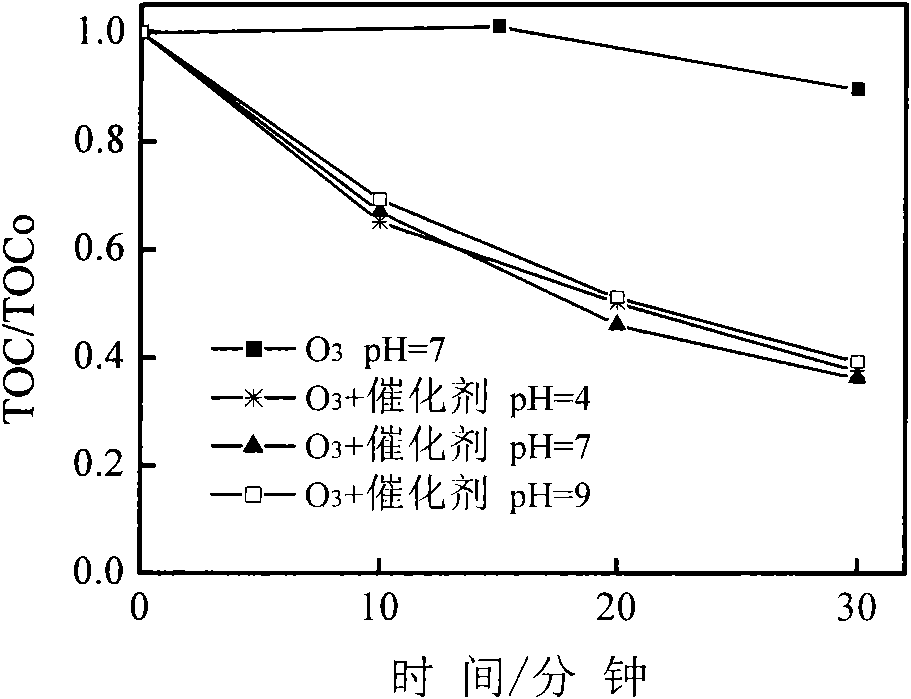
[0021] figure 1 The curves of TOC change with time for the degradation of organic pollutants by catalytic ozonation oxidation of honeycomb ceramic materials under different pH conditions are given. It can be seen from the figure that at pH=7, the removal effect of ozone oxidation alone on 2,4-dichlorophenoxyacetic acid is not obvious (the removal rate of TOC within 30 minutes is only 10.5%), and the removal of TOC after adding honeycomb ceramic catalyst The rate has reached about 63%, which is 53% h...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com