Benzoate derivatives, preparation method and application
A technology of derivatives, benzoic acid, applied in the fields of benzoic acid ester derivatives and preparation and application, can solve the problems such as no problems, and achieve the effects of simple operation, enhanced memory, and reasonable design
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
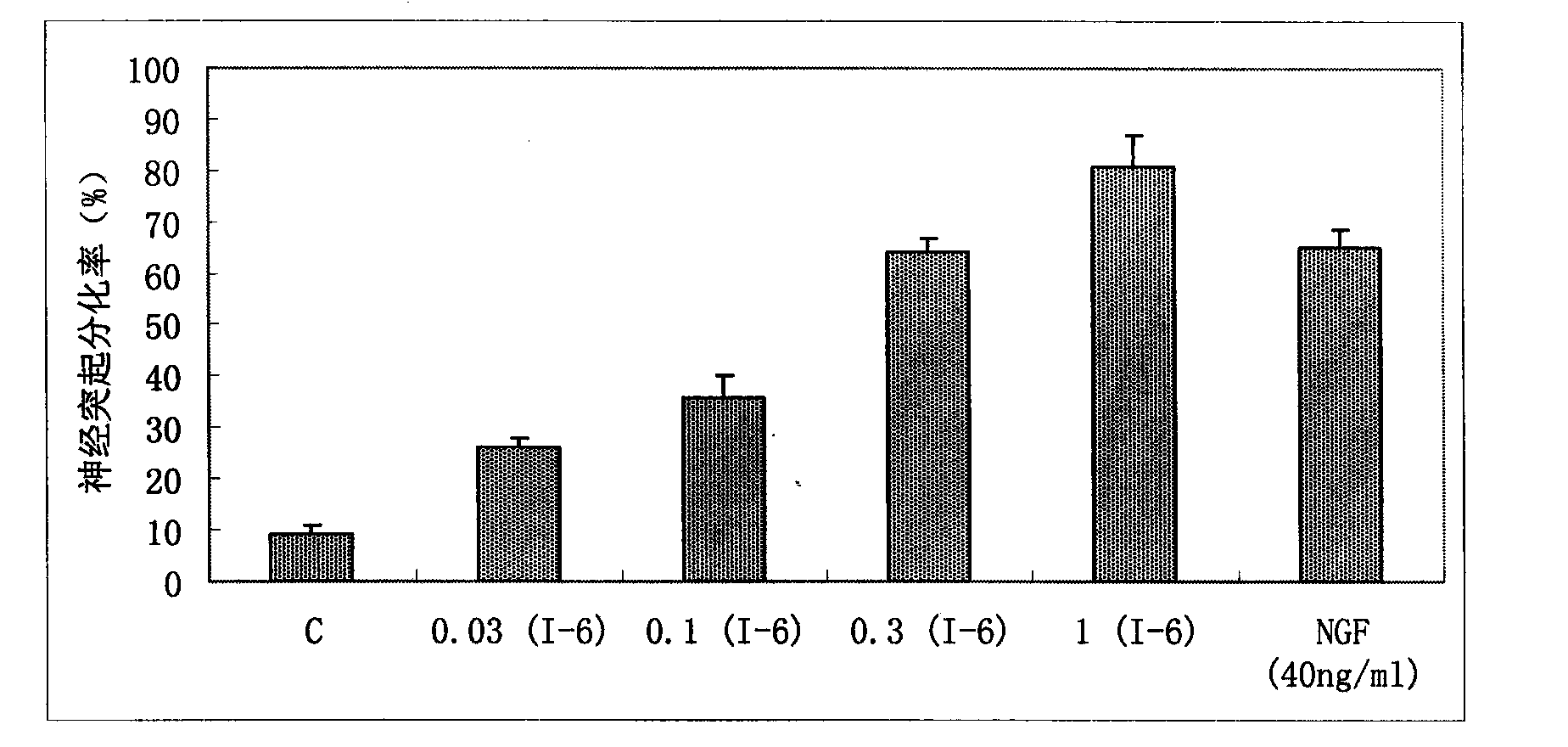
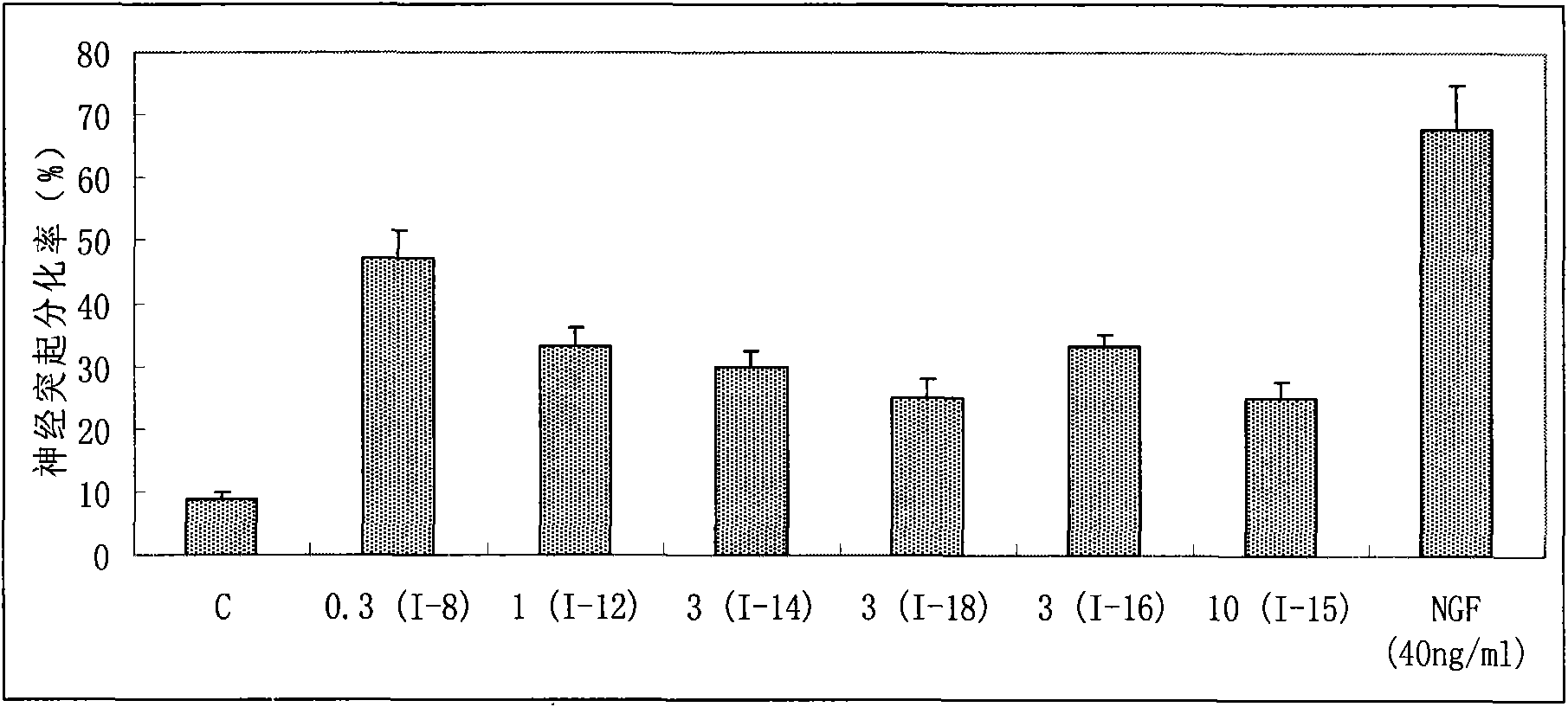
Image
Examples
Embodiment 1
[0042] Compound I-1: Ethyl 2,3-dihydroxybenzoate
[0043] Put (154mg, 1mmol) 2,3-dihydroxybenzoic acid and 10ml of ethanol in a 25ml round bottom flask, cool to 0°C, add 2-3 drops of concentrated sulfuric acid dropwise, and stir at reflux for 24h. The reaction was followed by thin layer chromatography (developing solvent: n-hexane / ethyl acetate, 5 / 1, V / V). After the reaction stopped, the ethanol was distilled to obtain a crude product of 390 mg. Silica gel column chromatography (developing solvent: n-hexane / Ethyl acetate, 5 / 1, V / V) to obtain 180 mg of white solid, yield: 99%. 1 H NMR(500MHz, CDCl 3 )δ: 11.00 (s, 1H, benzene 2-OH), 7.38 (dd, 1H, J=1.5, 8.0 Hz, benzene H-6), 7.12 (dd, 1H, J=1.0, 7.5 Hz, benzene H- 4), 6.80 (t, 1H, J = 8.0 Hz, benzene H-5), 5.66 (s, 1H, benzene 3-OH), 4.41 (q, 2H, J = 7.0 Hz), 1.42 (t, 3H, J=7.0Hz); MS(m / z): 182[M] + .
[0044] Compound I-2: Amyl 2,3-dihydroxybenzoate
[0045] The synthesis method is the same as that of compound I-1. The reaction ma...
Embodiment 2
[0083] Compound II-1: 1,2-phenylene icosanoate
[0084] The synthesis method is the same as that of compound I-4, and the reaction materials are: (110mg, 1mmol) catechol, (310mg, 1mmol) eicosanic acid, (250mg, 1.2mmol) dicyclohexylcarbodiimide, 15ml tetrahydrofuran to obtain white Solid 391mg, yield: 56%. 1 H NMR(500MHz, CDCl 3 )δ: 7.13~7.16(t,1H,J=7.5Hz), 7.09~7.11(d,1H,J=7.5Hz), 7.02~7.04(d,1H,J=7.5Hz), 6.92~6.95(t , 1H, J=7.5Hz), 2.63(t, 2H, J=6.5Hz), 2.42(t, 2H, J=6.5Hz), 1.96~1.99(m,12H), 1.17~1.42(m,60H) , 0.89 (bs, 6H); MS (m / z): 699 [M] + .
Embodiment 3
[0086] Compound III-1: 1,4-bis(tetradecyloxy)benzene
[0087] Under the protection of nitrogen, put (110mg, 1mmol) hydroquinone, (280mg, 5mmol) potassium hydroxide, 10ml N, N-dimethylformamide in a 25ml round bottom flask, and add (830mg, 3mmol) dropwise Bromotetradecane. Stir at room temperature overnight. After the reaction stops, the system is poured into a large amount of distilled water, and a yellow solid is obtained by chromatography, filtered, washed with water and washed with 10% sodium hydroxide to obtain 111 mg of the product, yield: 22%. 1 H NMR(500MHz, CDCl 3 )δ: 6.76(bs, 4H), 3.75~3.80(t,4H, J=6.5Hz), 1.82(m,4H), 1.25~1.33(m,44H), 0.85(t,6H,J=7.0Hz ); MS(m / z): 502[M] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com