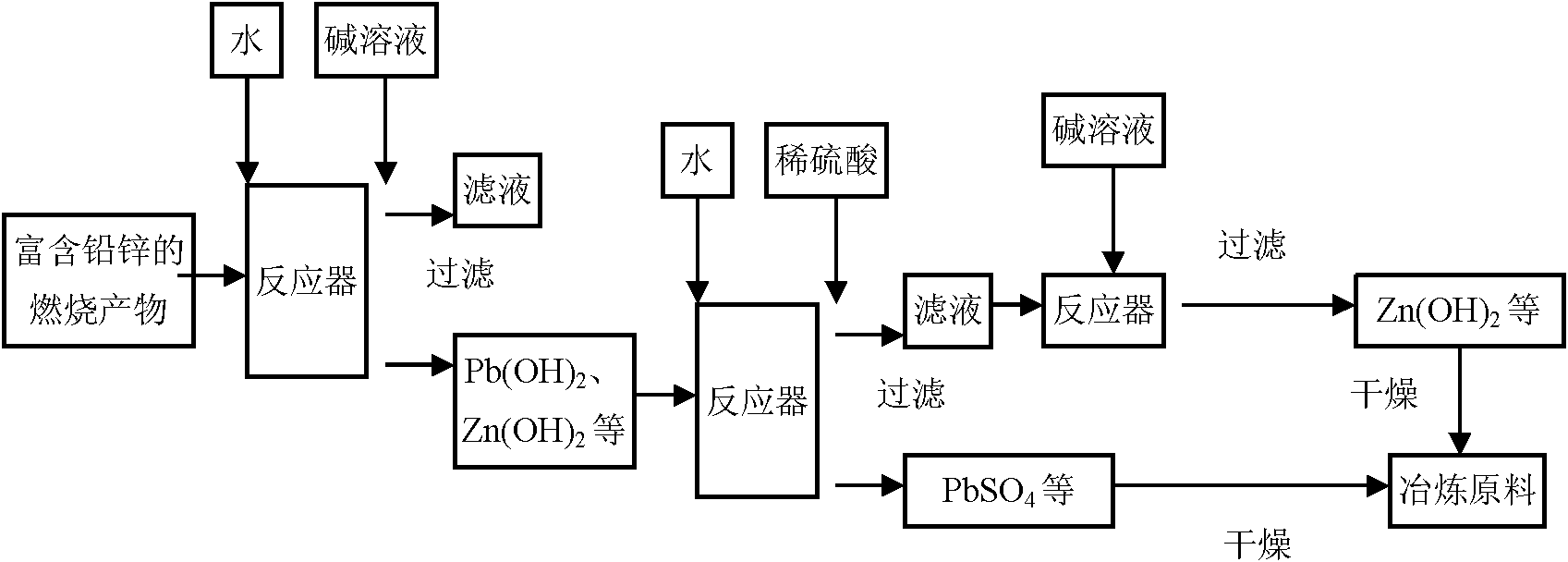
Wet chemical recovery method of lead and zinc in burning solid products
A recovery method and solid product technology, applied in the direction of solid waste removal, lead sulfate, zinc sulfate, etc., can solve the problems of uneconomical, high price, large energy consumption, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0027] The product of a waste incineration fly ash melting system is recovered lead and zinc according to the present invention, and the determination results of the original ash are shown in Table 1.
[0028] Table 1 Composition of molten fly ash 1
[0029]
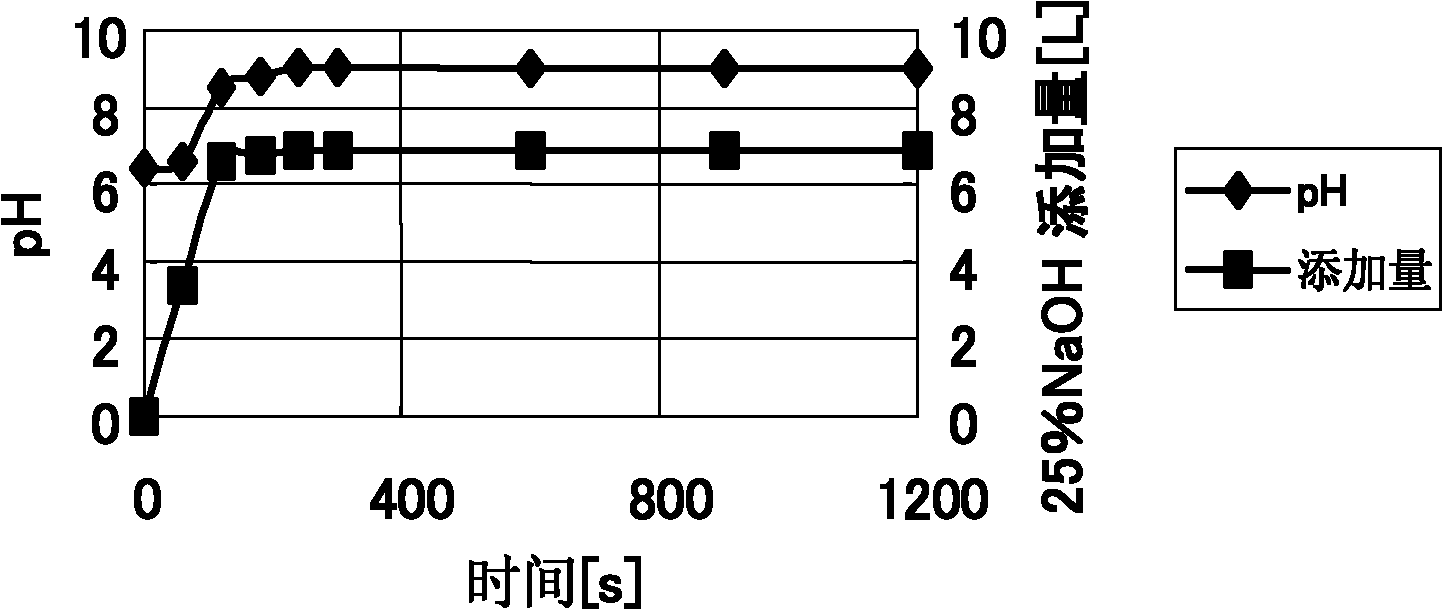
[0030] The first step is the dechlorination process. The main purpose of this step is to allow lead and zinc to enter the residue, and unwanted elements such as chlorine to enter the filtrate. Take 50 kg of molten fly ash, put it into a cylindrical reaction vessel, add 500 kg of water, stir with a mechanical stirrer at a speed of 300 rpm, and then add 7.848 L of NaOH solution with a mass concentration of 25% when stirring, and monitor the pH value of the solution while stirring. The reaction time is 20 minutes. The change of pH and alkali addition amount in the dechlorination process with time is shown in figure 2 . Precipitate then, filter, obtain wet residue quality 63.38kg, know that its dry basis quality is 3...
example 2
[0035] The product of a waste incineration fly ash melting system is recovered lead and zinc according to the present invention, and the determination results of the original ash are shown in Table 1.
[0036] Table 2 Composition of molten fly ash 2
[0037]
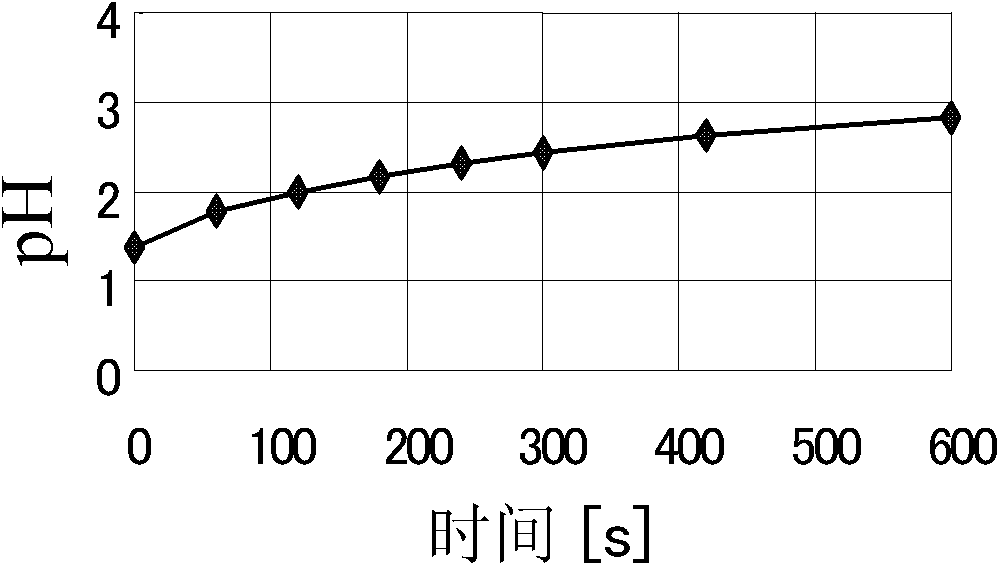
[0038] The first step is the dechlorination process. The main purpose of this step is to allow lead and zinc to enter the residue, and unwanted elements such as chlorine to enter the filtrate. Take 50kg of molten fly ash, put it into a cylindrical reaction vessel, add 500kg of water, stir with a mechanical stirrer at a speed of 300rpm, add 5.191L of 25% NaOH solution while stirring, and monitor the change of the pH value of the solution while stirring, and the reaction time is 5 minutes . Precipitate then, filter, obtain wet residue quality 117.9kg, know that its dry basis quality is 29.43kg by measuring moisture content. After testing the components of the filtrate and residue, the material balance calculation res...
example 3-13
[0043] The fly ash used in Example 1 is processed by this method, and the processing results under different operating parameters are shown in the table below.
[0044]
[0045]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com