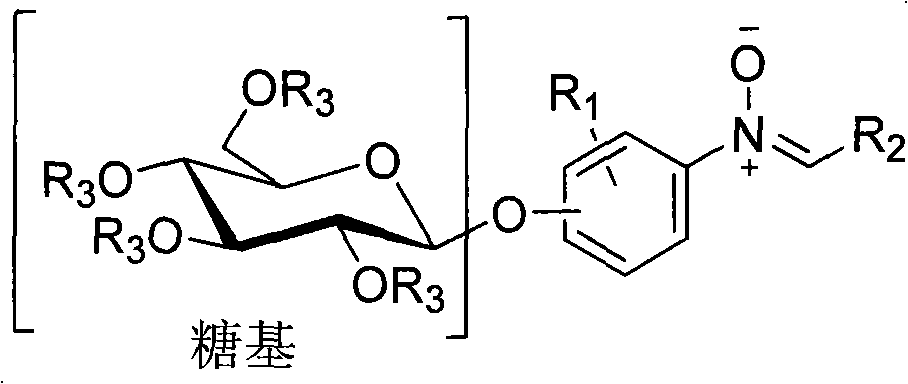
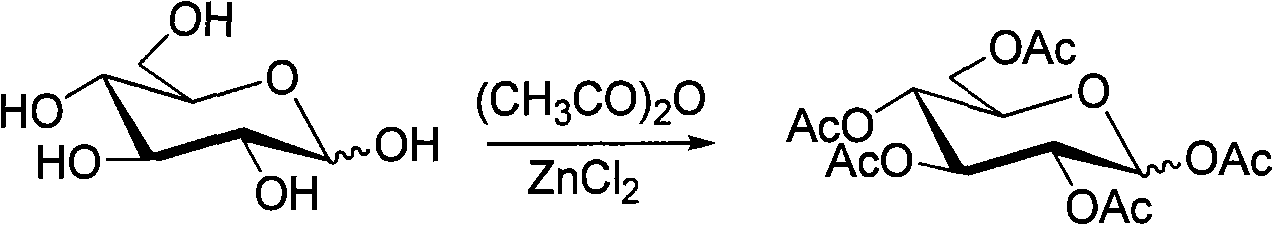O-glycoside nitrone compound and synthetic method thereof
A technology for glycoside nitrones and compounds is applied in the field of O-glycoside nitrones and their synthesis, which can solve the problems of limited wide application, poor absorption capacity of the body with toxic and side effects, etc., and achieves extended application range, cheap raw materials, and enhanced activity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1: Synthesis of C-[4-hydroxybenzaldehyde-2,3,4,6-tetraacetyl-O-β-(D)-glucoside]-N-phenylnitrone:
[0035] (1) Synthesis of 1,2,3,4,6-pentaacetyl-β-(D)-glucopyranose
[0036] Zinc chloride (3.7mmol) was added in batches to a round bottom flask containing glucose (14mmol) and acetic anhydride (123mmol), reacted at 100°C for 1 hour, cooled, the mixture was poured into ice water and stirred fully, white The precipitate is formed, filtered, washed with cold water, and recrystallized with ethanol to obtain white crystals. Yield: 52%. The reaction equation is as follows:
[0037]
[0038] (2) Synthesis of 2,3,4,6-tetraacetyl-β-(D)-bromoglucopyranose
[0039] Red phosphorus (29 mmol) was added to a 100 ml round bottom flask containing acetic acid (15 ml) and stirred vigorously for 5 minutes. Then liquid bromine (34mmol) was slowly added dropwise to the reaction flask, and the temperature was controlled between 15°C and 25°C. After the dropwise addition, continued stirring unt...
Embodiment 2
[0048] Example 2: Synthesis of C-[2-hydroxybenzaldehyde-2,3,4,6-tetraacetyl-O-β-(D)-glucoside]-N-phenylnitrone
[0049] (1) Synthesis of 1,2,3,4,6-pentaacetyl-β-(D)-glucopyranose
[0050] Same as Example 1 step (1).
[0051] (2) Synthesis of 2,3,4,6-tetraacetyl-β-(D)-pyran bromoglucose
[0052] Same as step (2) of Example 1.
[0053] (3) Synthesis of 2-hydroxybenzaldehyde-2,3,4,6-tetraacetyl-O-β-(D)-glucopyranoside
[0054] In a 100 ml three-necked flask containing tetrabutylammonium chloride (4.3 mmol), 15 ml of chloroform and water were added to dissolve them. Dissolve in KCO 3 O-Hydroxybenzaldehyde in aqueous solution (containing KCO 3 21.7mmol, water 10ml, o-hydroxybenzaldehyde 8.7mmol) and bromosugar solution in chloroform (6.08ml chloroform, 7.3mmol bromosugar prepared in step (2) above) were added dropwise to the reaction flask at the same time, and the temperature was controlled. At 58°C, TLC detected the reaction to completion (about 3-6h), then the reaction solution was coole...
Embodiment 3
[0060] Example 3, C-[4-hydroxy-3-methoxybenzaldehyde-2,3,4,6-tetraacetyl-O-β-(D)-glucoside]-N-phenylnitrone synthesis
[0061] (1) Synthesis of 1,2,3,4,6-pentaacetyl-β-(D)-glucopyranose
[0062] Same as Example 1 step (1).
[0063] (2) Synthesis of 2,3,4,6-tetraacetyl-β-(D)-pyran bromoglucose
[0064] Same as step (2) of Example 1.
[0065] (3) Synthesis of 4-hydroxy-3-methoxybenzaldehyde-2,3,4,6-tetraacetyl-O-β-(D)-glucopyranoside
[0066] In a 100 ml three-necked flask containing tetrabutylammonium chloride (4.3 mmol), 15 ml of chloroform and water were added to dissolve them. Dissolve in K 2 CO 3 Vanillin in aqueous solution (K 2 CO 3 21.7mmol, water 10ml, vanillin 8.7mmol) and bromosugar solution in chloroform (6.08ml chloroform, 7.3mmol for bromosugar prepared in step (2)) were added dropwise to the reaction flask at the same time, the temperature was controlled at 58℃ , TLC detects that the reaction is complete (about 3-6h), then the reaction solution is cooled to room temperatu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com