Recombinant beta-lactamase-RGD-fusion protein and application thereof in medicine
A fusion protein, lactamase technology, applied in recombinant DNA technology, drug combinations, peptide/protein components, etc., can solve problems such as toxic side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
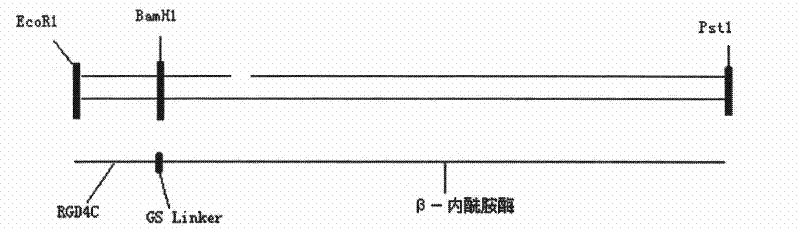
[0046] Construction of β-lactamase-RGD4C fusion gene:
[0047] (1) According to the amino acid sequence of the fusion protein, use OptimumGene TM The gene optimization software optimizes the DNA sequence of the protein, and the obtained DNA sequence is as follows:
[0048] TGTGACTGTCGTGGCGATTGTTTCTGTGGCGGTGGCGGCAGCACACCAGTTAGTGAAAAACAACTGGCAGAGGT
[0049] GGTGGCGAACACGATTACCCCACTGATGGCTGCTCAATCCGTTCCAGGTATGGCAGTGGCAGTTATTTTATCAGG
[0050] GCAAACCGCATTACTATAACTTTCGGGAAAGCGGATATCGCGGCCAACAAACCAGTCACTCCACAAACCCTGTTC
[0051] GAACTGGGGAGTATTTCCAAAACGTTTACGGGTGTACTGGGCGGCGACGCTATTGCCCGCGGGGAAATTAGTCT
[0052] GGACGATGCTGTAACCCGTTACTGGCCACAACTGACTGGGAAACAGTGGCAGGGTATCCGCATGCTGGACCTGG
[0053] CGACATACACAGCCGGGGGGCTGCCACTGCAGGTCCCAGATGAGGTTACTGACAACGCCTCTCTGCTGCGTTTT
[0054] TATCAGAACTGGCAACCACAATGGAAACCAGGCACAACACGCCTGTACGCAAATGCATCGATTGGTCTGTTTGG
[0055] CGCTCTGGCTGTTAAACCAAGCGGTATGCCGTATGAACAGGCAATGACGACCCGCGTTCTGAAACCACTGAAAC
[0056] TGGACCATACGTGGATCAACGTACCAAAAAGCCGAA...
Embodiment 2
[0065] Expression of β-lactamase-RGD4C fusion protein:
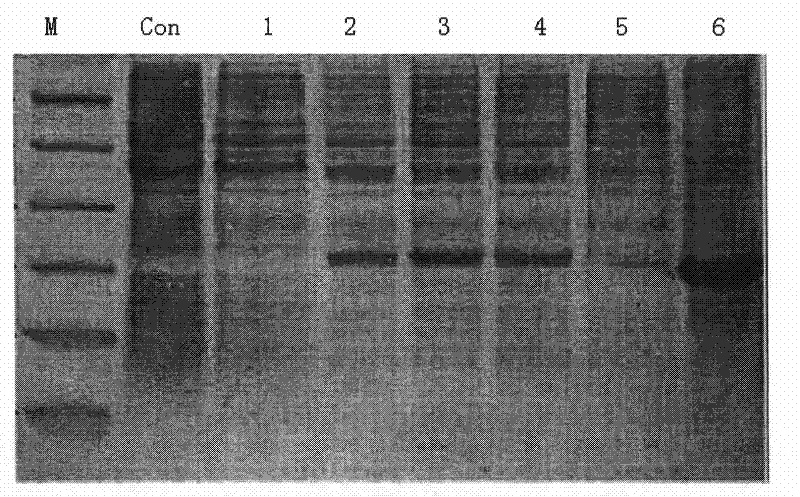
[0066] The β-lactamase-RGD4C fusion gene verified by sequencing was cloned into the vector pET30a(+) to transform the competent cell BL21(DE3) bacteria, and a single bacterium was picked and inoculated in LB liquid medium, cultured with shaking at 37°C overnight, and then 1 : 100 inoculated in liquid medium containing 25-170 μg / ml chloramphenicol, when OD 600 When reaching about 0.5-1.0, add inducer IPTG to induce protein expression, and the concentration of IPTG is 0.5mM. After continuing to cultivate for 2 hours, centrifuge at 15000 g for 10 min. After being purified by nickel column affinity chromatography, the fusion protein β-lactamase-RGD4C was obtained by freeze-drying, and the induced expression fusion protein was subjected to SDS-polyacrylamide gel electrophoresis.
[0067] The special feature of the present invention is that the time and temperature for inducing expression can be adjusted according to the act...
Embodiment 3
[0069] Enzyme activity and cell adhesion activity assay of β-lactamase-RGD4C fusion protein:
[0070] Dilute the purified β-lactamase-RGD4C fusion protein with coating solution so that the final concentrations are 30,000, 20,000, 10,000, 6,000, 2,000, 500, and 200 ng / ml, and add to 96 wells at a volume of 100 μl per well Cell culture plates were coated overnight at 4°C. After washing the plate three times with 0.9% NaCl, add 200 μl of blocking solution to each well, block at 37° C. for 2 hours, and then wash the plate three times with 0.9% NaCl. After the MCF-7 breast cancer cells were washed three times with 0.9% NaCl, the cells were resuspended with incomplete medium. Add 100 μl of cell suspension to each well, incubate for 1 hr, wash the plate, and remove non-adherent cells. Cells adhering to the culture wells were fixed with fixative solution, stained with 0.5% crystal violet, observed cell adhesion with an inverted microscope and photographed. The results are shown in ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com