New method for preparing alverine citrate
A technology of alverine citrate and phenylpropanol, which is applied in the preparation of amino-substituted functional groups, the preparation of amino compounds from amines, organic chemistry, etc., can solve problems such as toxicity and difficult reaction conditions, and achieve mild reaction conditions and easy operation. Ease of the process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
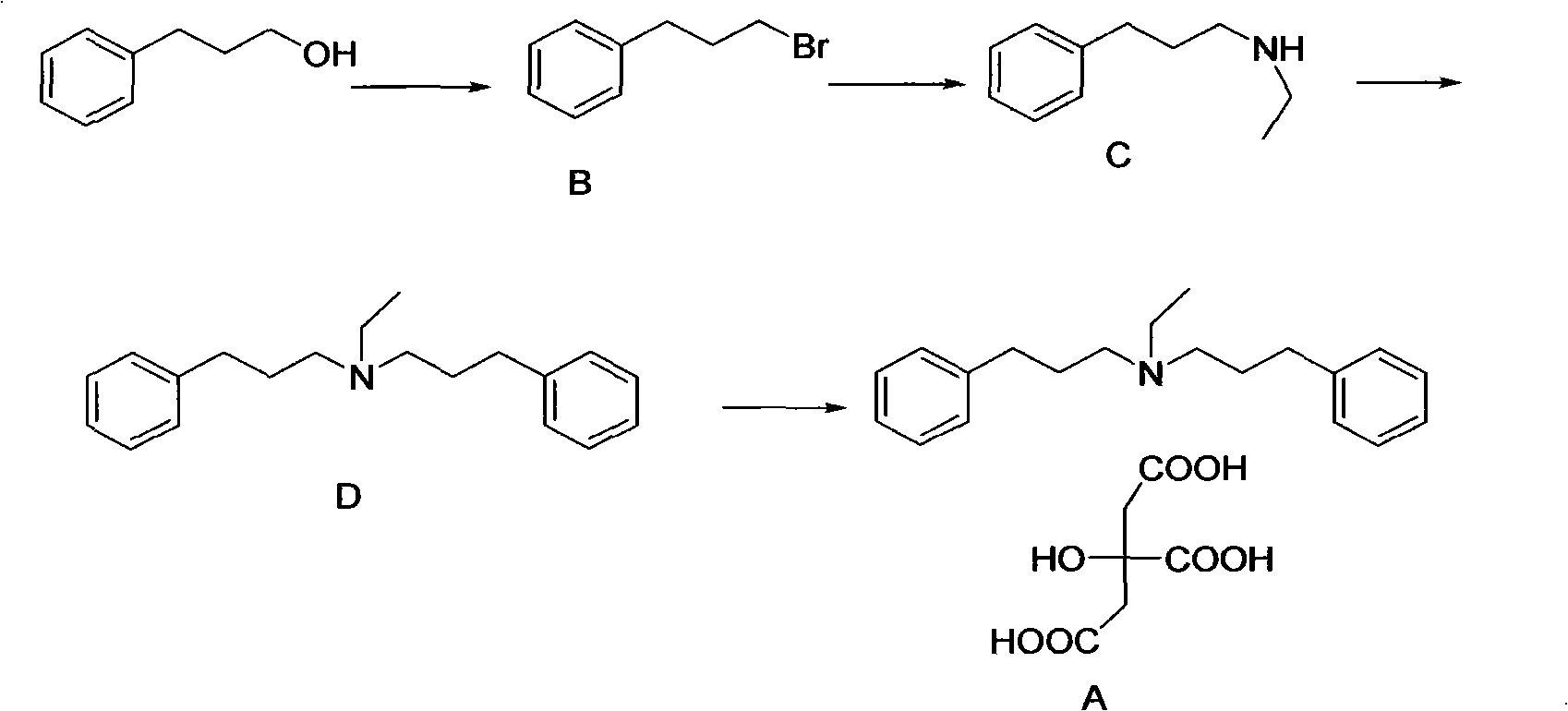
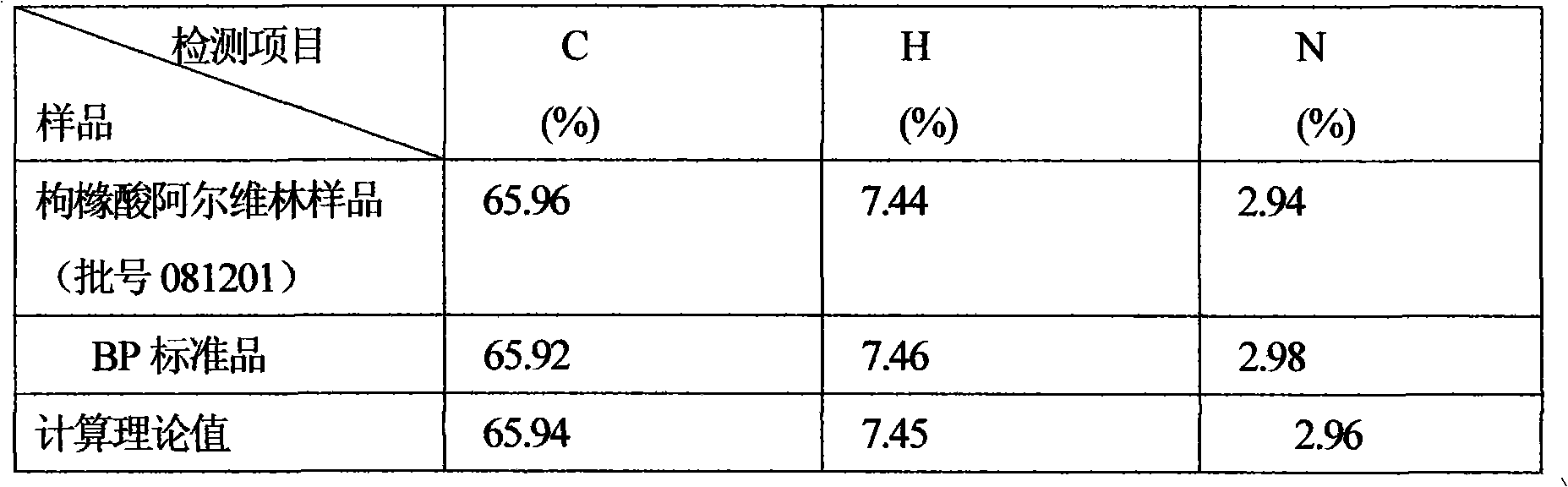
[0012] Embodiment: the preparation of alverine citrate
[0013] The first step: the preparation of 3-phenylbromopropane
[0014] Add 50ml of water into a 250ml flask, carefully add 70ml of concentrated sulfuric acid, mix well, cool to room temperature, then add 68g of phenylpropanol and 75g of sodium bromide in turn, heat to reflux, the reaction is over, separate the organic layer, and the organic layer in turn Use 100ml of water, 5ml of concentrated sulfuric acid, 100ml of water, and 100ml of saturated sodium bicarbonate solution. Then washed three times with 100 ml of water each time to obtain 80 g of orange-red oily liquid with a yield of 80%, and no further purification was required.
[0015] Step 2: Preparation of Bethamphetamine
[0016] Add 0.05mol (9.9g) of 3-phenylbromopropane dropwise to a 250ml flask filled with 100ml of ethylamine solution under ice bath conditions, react for 4h under ice bath, and then continue to react at room temperature, followed by TLC, the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com