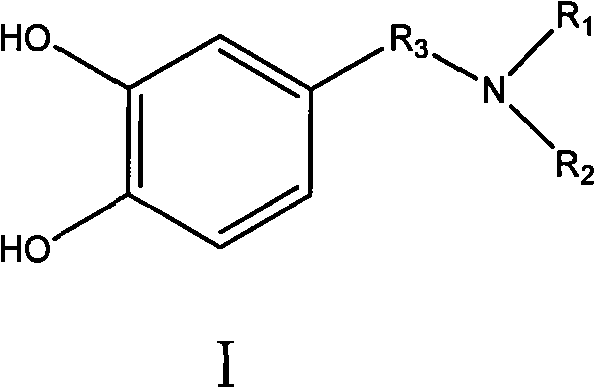
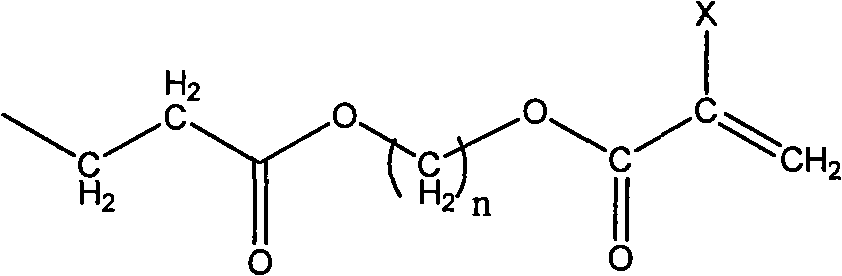
Photo-curing monomer with ortho-phenolic hydroxyl structure, preparation method and bond thereof
A technology of o-phenolic hydroxyl and light curing, which is applied in the direction of non-polymer organic compound adhesives, cyanide reaction preparation, chemical instruments and methods, etc., to achieve the effects of wide practicability, low preparation cost, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041]
[0042] Dissolve 10 g of dopamine hydrochloride in a mixed solution of 200 ml deionized water and 40 ml ethanol, fill with nitrogen for 20 min, then add 19.8 g of acryloyloxyethyl methacrylate, and 6 g of triethylamine, at 35 The reaction was carried out at ℃ for 6 hours until the solution was clear. The reaction solution was extracted with a mixed solution of 160ml of ethyl acetate and 40ml of ethanol, and then the ethyl acetate layer was evaporated to remove the solvent with a rotary evaporator to obtain 18.6g of the product. NMR 1 H-NMR identification, 1 H-NMR (DMSO, 600MHz): δ (ppm): 1.93 (CH 3 ); 2.35, 2.65, 2.69, 2.75, 4.36, 4.43 (CH 2 ); 6.42, 6.51 (CH, Ar); 5.58, 6.15 (=CH 2 )
[0043]The product is diluted with acetone to form a solution with a weight percentage of 75%, adding 2% of the total weight of UV photoinitiator 2959, and initiating polymerization under a point light source with a light intensity of 30mW / cm2. All bonding performance tests are i...
Embodiment 2
[0047]
[0048] Dissolve 16g of methyldopamine in 200ml of de-ethanol, fill with nitrogen for 20min, then add 19.8g of acryloyloxybutyl acrylate, react at 35°C for 14 hours until the solution is clear. Ethanol was evaporated with a rotary evaporator to remove the solvent, and the crude product was rinsed with a developing solvent composed of 7:4 dichloromethane:methanol, and the product was subjected to column chromatography to collect the initial fraction. The solvent was distilled off again to obtain 9.6 g of the product. NMR 1 H-NMR identification, 1 H-NMR (DMSO, 600MHz): δ (ppm): 2.27 (CH 3 ); 2.35, 2.65, 2.69, 2.75, 4.15, 4.08 (CH 2 ); 6.42, 6.51 (CH, Ar); 5.80, 6.43 (=CH 2 ); 6.05 (-CH=).
[0049] The product is added with 2% of the total weight of ultraviolet photoinitiator 2959, and the polymerization is initiated under a point light source with a light intensity of 30mW / cm2. All bonding performance tests are all in accordance with the ASTMF225-03 standard, and...
Embodiment 3
[0052]
[0053] Dissolve 9.5g of levodopa in 300ml of ethanol for 20 minutes and add to the reaction system, then add 16.8g of acryloyloxydecyl methacrylate into the reaction system until the solution is uniform. The reaction was kept at 35° C., and after 18 hours of reaction, the by-product was precipitated with cyclohexane, and the solvent was evaporated by rotary evaporation to obtain a crude product. The product was eluted with a developing solvent of ethyl acetate:n-hexane with a ratio of 9:2, and the product was subjected to column chromatography to collect the initial fraction. The solvent was distilled off again to obtain 13.4 g of the product. NMR 1 H-NMR identification, 1 H-NMR (DMSO, 600MHz): δ (ppm): 1.94 (CH 3 ); 2.35, 2.65, 2.69, 2.78, 3.03, 4.15, 4.08 (CH 2 ); 3.88 (CH) 6.42, 6.51 (CH, Ar); 5.58, 6.15 (=CH 2 ).
[0054] The product is added with 2% of the total weight of UV photoinitiator 2959, and the polymerization is initiated under a point light sou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com