Isolated cell mass
A cell group, cell technology, applied in the field of cell groups
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0153] Preparation of Antibody, Selectin-IgG Fusion Protein and α1,3-Fucosyltransferase
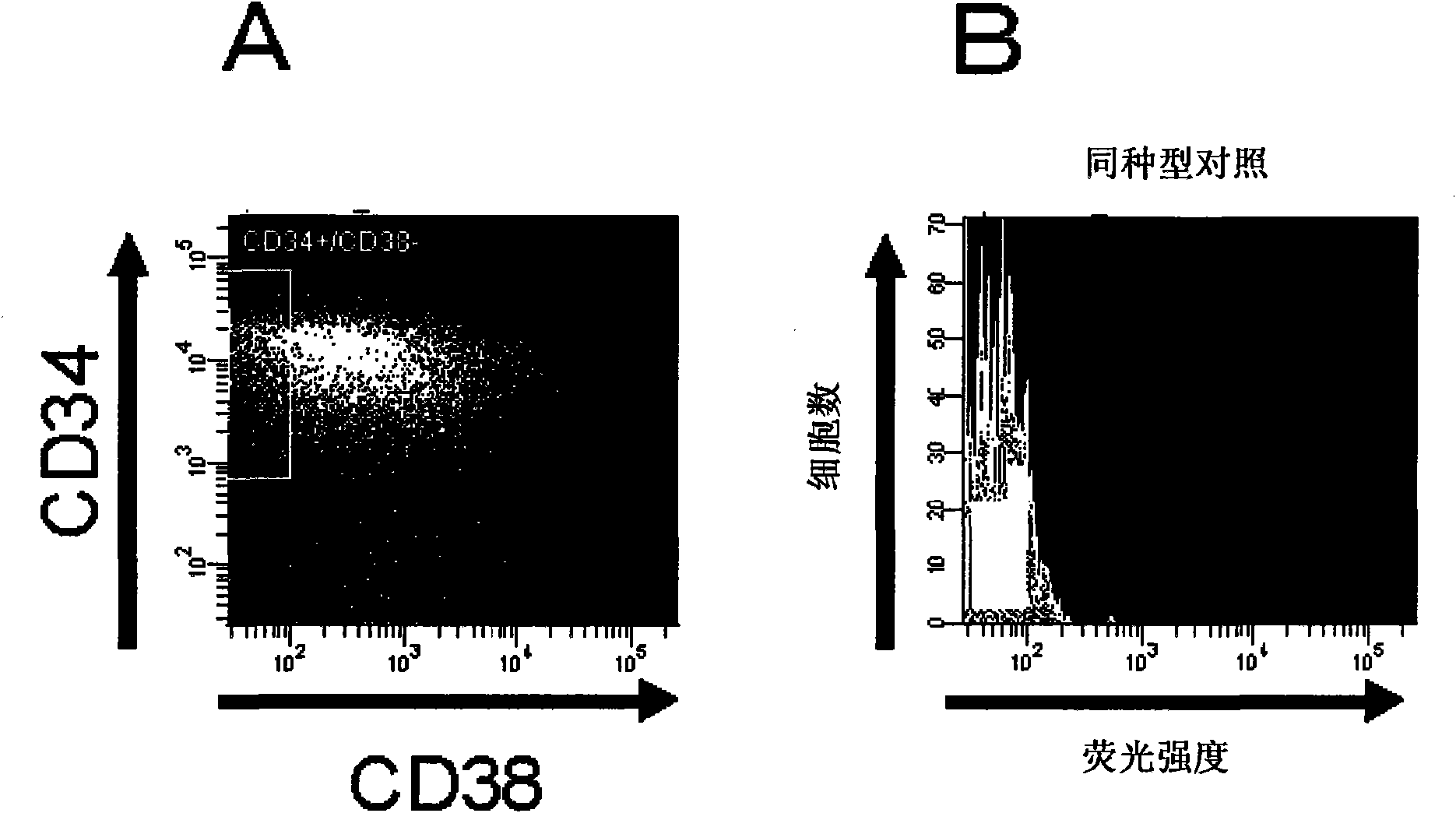
[0154] Fluorescein isothiocyanate (FITC)-labeled anti-human Lewis X antibody (HI 98), FITC-labeled anti-mouse IgM antibody (II / 41), IgM isotype control antibody (11E10), Phycoerythrin Protein (PE)-labeled anti-human CD38 antibody (HIT 2), PE-labeled anti-mouse CD45 antibody (30-F11), PE-labeled anti-mouse TER-119 antibody (TER-119) and Allosa Procyanin (APC)-labeled human CD34 antibody (581 ) was purchased from BectonDickinson Pharmingen. FITC-labeled and APC-labeled human CD45 antibodies (HI30) were purchased from eBioscience. FITC-labeled anti-human IgG antibody was purchased from DAKOCytomation.
[0155] As an anti-human sialyl Lewis X sugar chain antibody (KM 93), an antibody collected from a hybridoma culture supernatant was used [Hanai et al., Cancer Research, 46 , 4438-4443 (1986)].
[0156] A fusion protein of human E-selectin and human IgG constant region (Fc) (hereinafter ...
Embodiment 2
[0206] Migration of Mesenchymal Stem Cells to the Liver in a Hepatitis Model
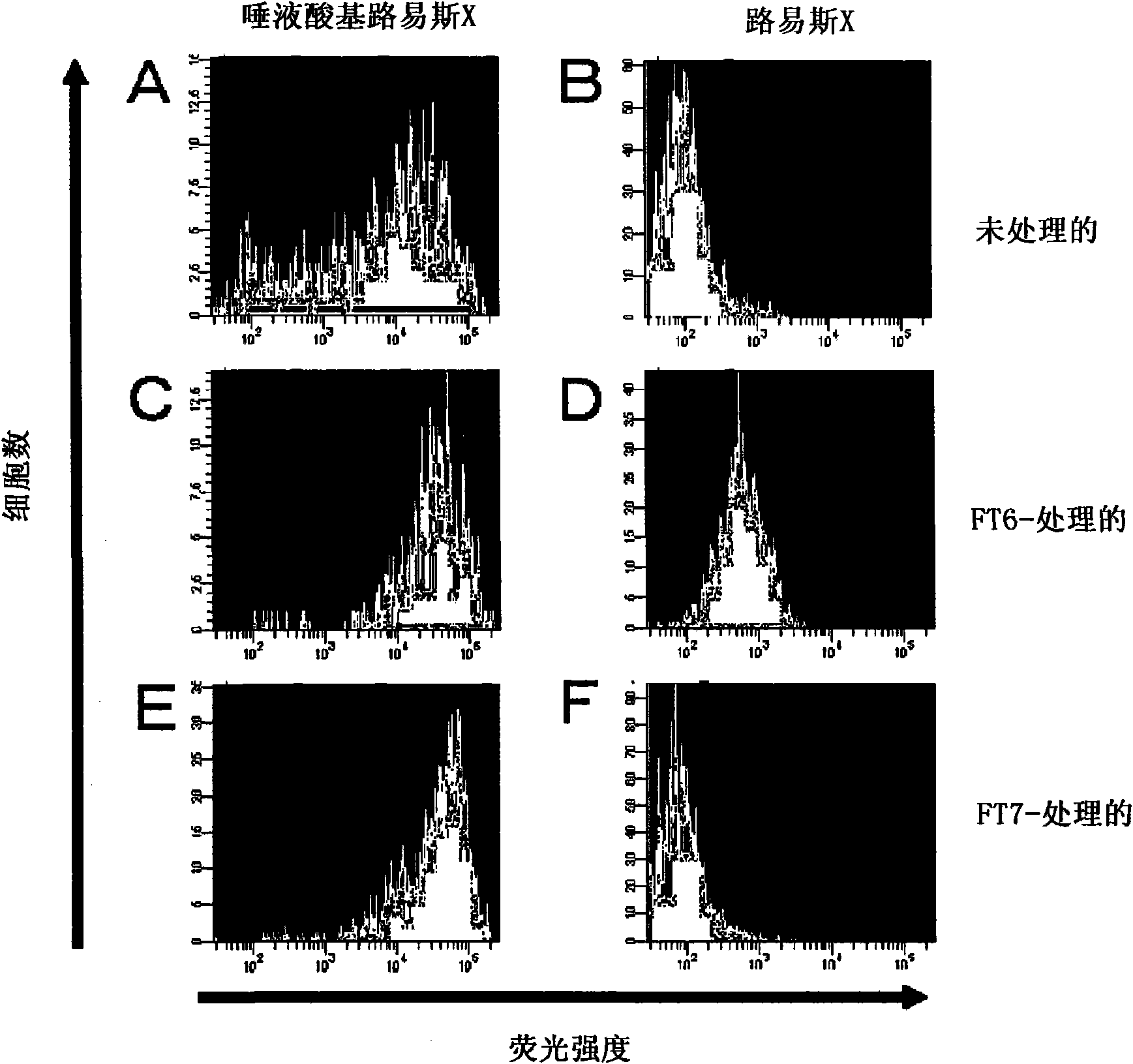
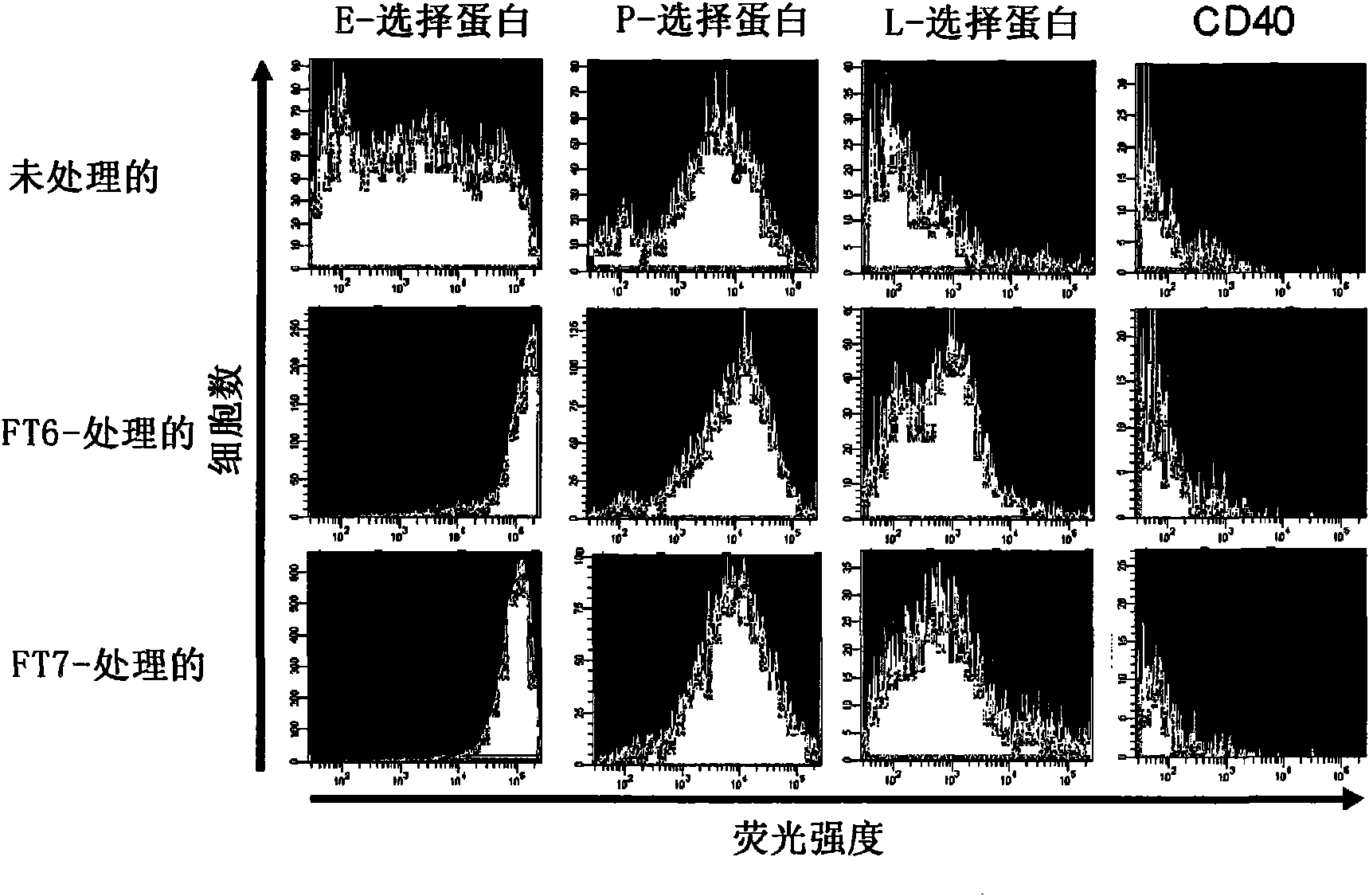
[0207] 1. Lewis X sugar chains and sialyl Lewis X sugar chains in human mesenchymal stem cells expression analysis of
[0208] Human mesenchymal stem cells (hereinafter referred to as hMSCs) were purchased from CAMBREX. Place hMSCs in CO 2 In an incubator, use MSCGM medium (manufactured by CAMBREX) at 37°C and 5% CO 2 cultured under conditions. After washing with phosphate-buffered saline (hereinafter referred to as PBS(-)), the cells were detached by adding TrypLE Select (manufactured by Invitrogen) and treated at 37°C for 2 minutes, and then by adding PBS containing 5% FBS (-) solution terminates the reaction. Cells were recovered by centrifugation at 1,000 rpm (rev / min) for 5 minutes, washed with PBS(-), and then 1-2×10 6 Each cell is suspended in 80-100 μ l of Hanks' balanced salt solution (HBSS) reaction solution [25mmol / l HEPES (manufactured by Gibco), 10mmol / lMnCl 2 (manufactured ...
Embodiment 3
[0222] Migration of Mesenchymal Stem Cells to the Lung in a Pneumonia Model
[0223] 1. Preparation of mouse carbon tetrachloride lung disease model
[0224] Five-week-old male BALB / c-nu / nu Slc mice (Japan SLC) were adaptively bred for 1 week. By diluting carbon tetrachloride (manufactured by Wako Pure Chemical Industries) 10 times with olive oil (manufactured by Wako Pure Chemical Industries), small amounts of carbon tetrachloride to BALB / c-nu / nu Slc were carried out at a dose of 1 ml / kg. Intraperitoneal administration of mice induced acute lung disease [Mizuguchi et al., BioFactors, 26 , 81-92 (2006)].
[0225] 2. hMSC transplantation into mouse carbon tetrachloride lung disease model and analysis
[0226] By the same method as in Example 2, FT 7 enzyme-treated hMSCs or negative control hMSCs were suspended in PBS(-) and adjusted to 2.5×10 6 cells / ml. 24 hours after administration of carbon tetrachloride, with 5 × 10 5 FT 7 enzyme-treated hMSCs or control hMSCs w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com