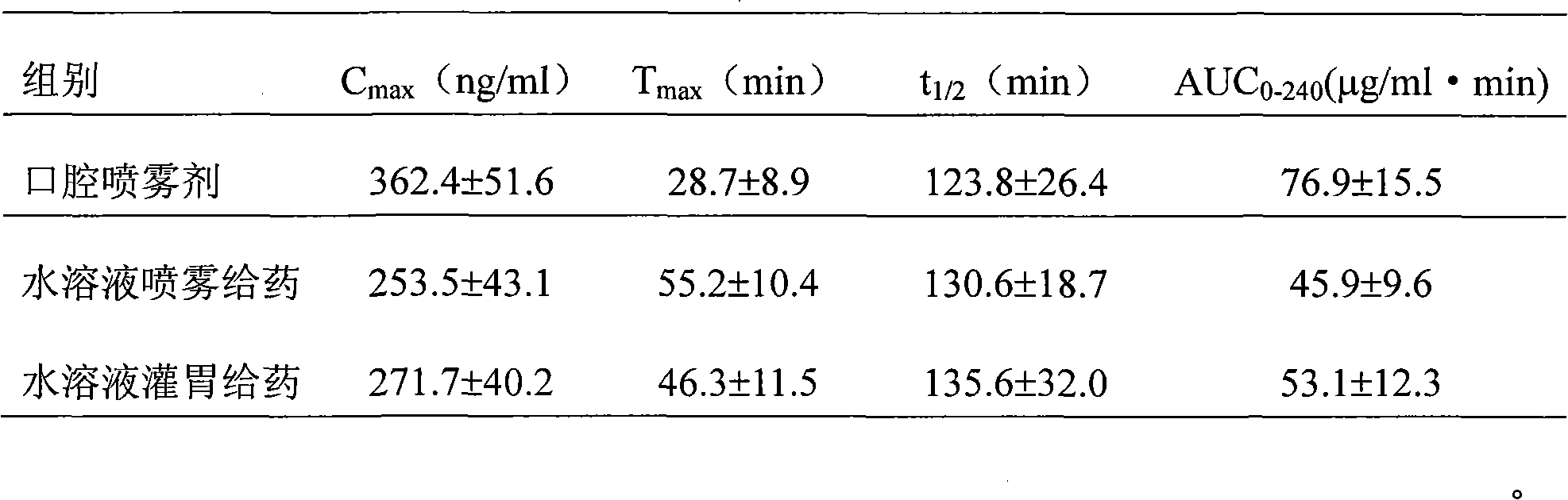
Rizatriptan benzoate oral spray
A technology of rizatriptan benzoate and oral spray, which is applied in the field of rizatriptan benzoate preparations, can solve the problems of low bioavailability and high production cost of freeze-drying process, and achieve high bioavailability and easy Effects of labor protection and ease of use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Formula: Rizatriptan Benzoate 7.3g
[0042] Sodium caprate 0.3g
[0043] Aspartame 0.5g
[0044] Ethanol 25g
[0045] Propylene glycol 10g
[0046] Menthol 0.4g
[0047] EDTA-2Na 0.05g
[0048] Sorbic acid 0.1g
[0049] Distilled water to 100ml
[0050] Preparation method: Take 7.3g of rizatriptan benzoate, add 25g of ethanol, 10g of propylene glycol and 50ml of distilled water, stir to dissolve. Add 0.3 g of sodium caprate, 0.5 g of aspartame, 0.05 g of EDTA-2Na, 0.1 g of sorbic acid, and 0.4 g of menthol. After dissolving, add distilled water and dilute to 100 ml. The solution was passed through a 0.22 μm microporous membrane, and filled in a spray bottle with a quantitative valve.
Embodiment 2
[0052] Formula: Rizatriptan Benzoate 3.6g
[0053] Water-soluble Azone 0.8g
[0054] Ethanol 10g
[0055] Propylene glycol 10g
[0056] Hypromellose 0.1g
[0057] Neotame 0.3g
[0058] Lemon essence 0.2g
[0059] EDTA-2Na 0.1g
[0060] Potassium sorbate 0.15g
[0061] Distilled water to 100ml
[0062] Preparation method: Dissolve 0.1g of hydroxypropylmethylcellulose in 70ml of pure water, add 10g of ethanol, 10g of propylene glycol, and 3.6g of rizatriptan benzoate, and stir to dissolve. Add 0.8g of water-soluble azone, 0.6g of neotame, 0.2g of lemon essence, 0.1g of EDTA-2Na, and 0.15g of potassium sorbate. After dissolving, add distilled water to make the volume to 100ml. The solution was passed through a 0.22 μm microporous membrane, and filled in a spray bottle with a quantitative valve.
Embodiment 3
[0064] Formula: Rizatriptan Benzoate 14.5g
[0065] Citric acid 2g
[0066] Propylene glycol 15g
[0067] Ethanol 25g
[0068] Caprylic Capric Macrogol Glycerides 0.5g
[0069] Sucralose 0.3g
[0070] Menthol 0.4g
[0071] EDTA-2Na 0.1g
[0072] Methylparaben 0.15g
[0073] Distilled water to 100ml
[0074] Preparation method: Dissolve 2 g of citric acid in 15 g of ethanol, 15 g of propylene glycol and 50 g of water, add 14.5 g of rizatriptan benzoate, and stir to dissolve. Add 0.3g of sucralose, 0.15g of methylparaben, and 0.1g of EDTA-2Na. After dissolving, add 10g of ethanol solution in which 0.4g of menthol and 0.5g of caprylic capric acid macrogolglyceride are dissolved, mix well, and add Distilled water to make up to 100ml. The solution was passed through a 0.22 μm microporous membrane, and filled in a spray bottle with a quantitative valve.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com