New type slightly soluble oral medicine self-emulsification preparation and preparation method thereof
An insoluble drug and self-emulsifying technology, applied in the field of medicine, can solve the problems of industrialization of organic solvent residues, drug precipitation, poor compliance, etc., achieve high drug solubility and dissolution, easy filling and use, and good water dispersibility. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
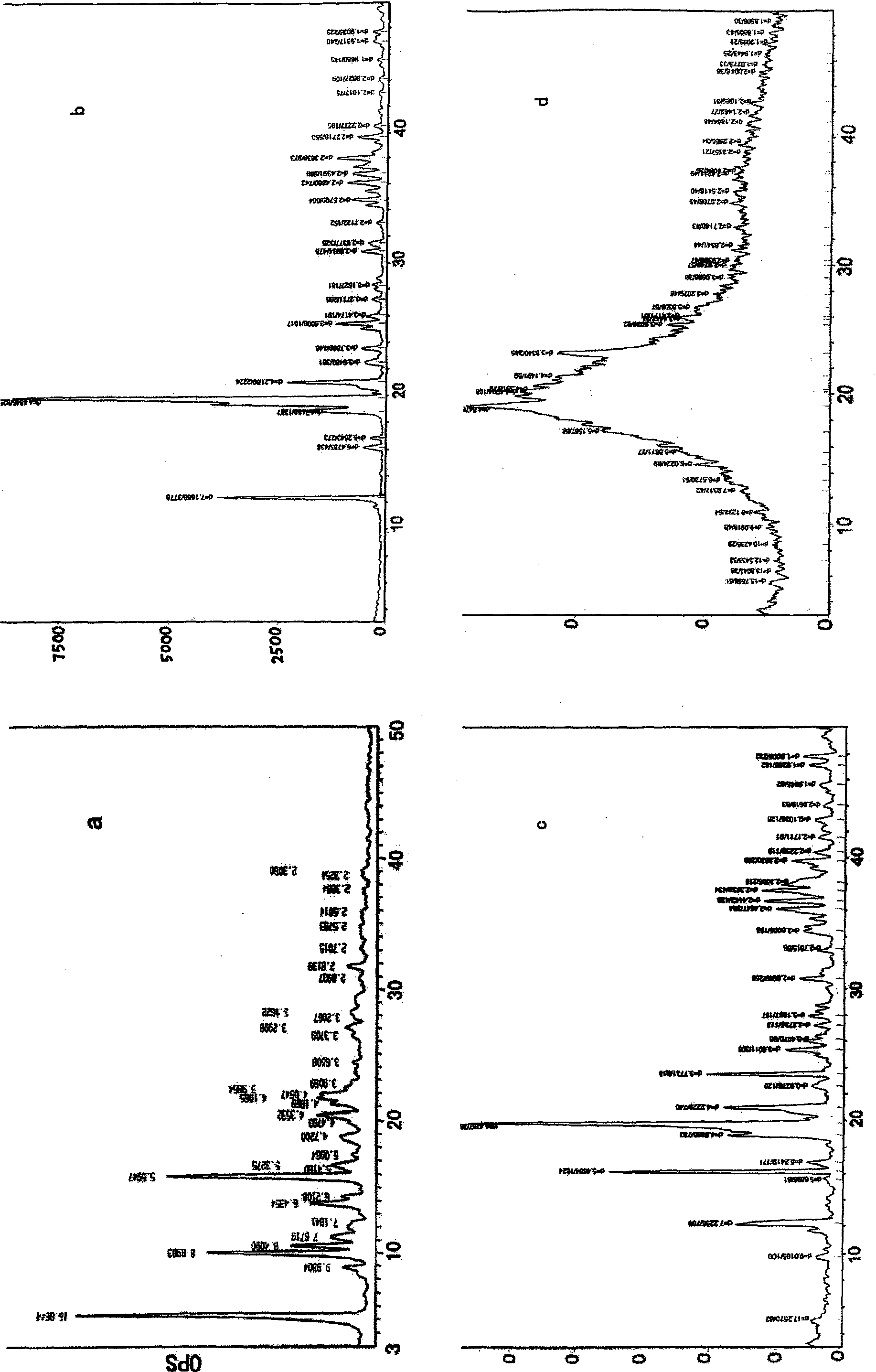
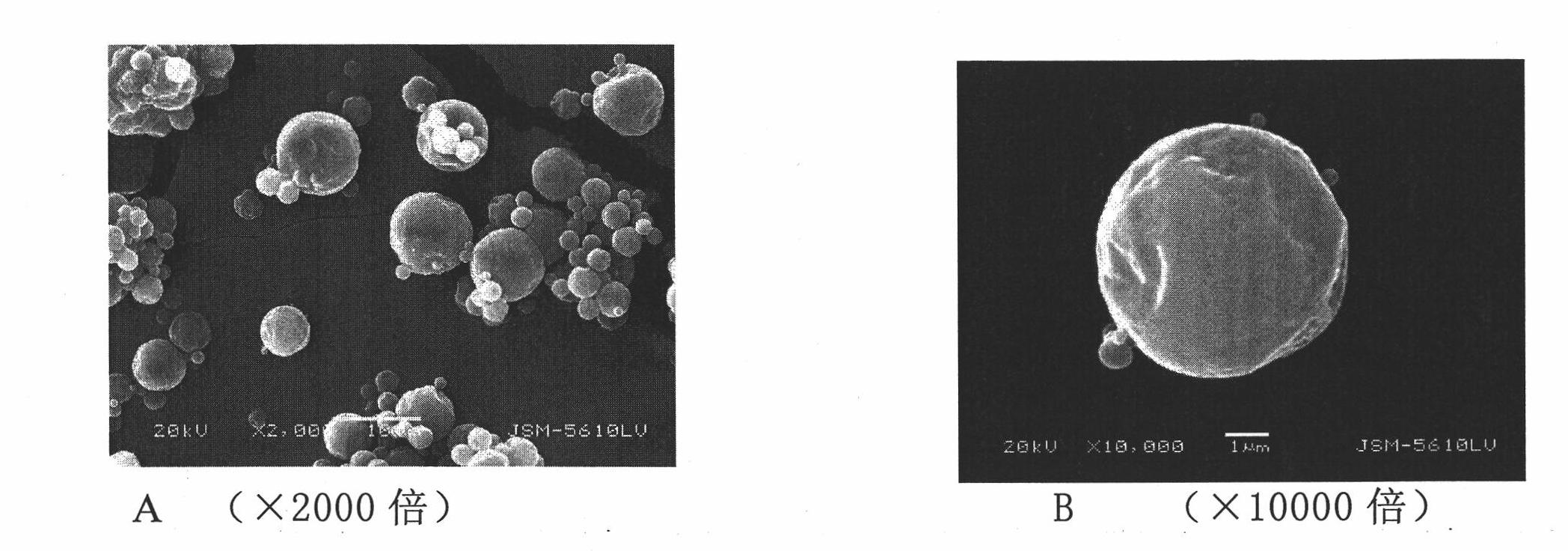
Image
Examples
Embodiment 1
[0060]Prescription composition:
[0061] Docetaxel 20g
[0062] Labrafac (oil phase) 90g
[0063] Cremophor RH40 (surfactant) 150g
[0064] Transcutol P (co-surfactant) 60g
[0065] HPMC K100-LV (supersaturation-promoting substance) 9g
[0066] Lactose (solid carrier) 400g
[0067]
[0068] Made a total of 1000 hard capsules
[0069] Preparation Process:
[0070] Weigh the oil phase, surfactant, and co-surfactant of the prescribed amount, and stir evenly to obtain a uniform clear oily liquid (that is, a blank liquid self-emulsifying preparation), and add the prescribed amount of medicine to the above-mentioned clear oily liquid with stirring at room temperature , stir and mix for about 1 hour to fully dissolve the drug (that is, to obtain a drug-containing liquid self-emulsifying preparation), then add supersaturated substances into it, and stir well, to obtain a drug-containing liquid su...
Embodiment 2
[0099] Prescription composition:
[0100] Docetaxel 20g
[0101] Labrafac (oil phase) 10g
[0102] Cremophor RH40 (surfactant) 15g
[0103] Transcutol P (co-surfactant) 5g
[0104] HPMC K100-LV (supersaturation-promoting substance) 2g
[0105] PEG6000 (solid carrier) 200g
[0106]
[0107]Made a total of 1000 hard capsules
[0108] Preparation Process:
[0109] Weigh the prescribed amount of oil phase, surfactant, co-surfactant and supersaturation-promoting substance, stir evenly, then add the prescribed amount of drug and appropriate amount of ethanol under stirring at room temperature, stir and mix for about 0.5h to fully dissolve the drug Another accurately weighed solid carrier PEG6000 was heated and melted on a (70±2)°C water bath, and under constant stirring, the ethanol solution of the above drug was poured into the molten carrier, and after the organic solvent was evaporated and removed, it was quickly moved to...
Embodiment 3
[0115] Prescription composition:
[0116] Paclitaxel 10g
[0117] Glyceryl linoleate (oil phase) 40g
[0118] Cremophor EL (surfactant) 50g
[0119] Transcutol P (co-surfactant) 10g
[0120] HPMC K100-LV (supersaturation-promoting substance) 2.5g
[0121] Dextran (solid carrier) 200g
[0122]
[0123] Made a total of 1000 hard capsules
[0124] Preparation Process:
[0125] Weigh the prescribed amount of oil phase, surfactant, and co-surfactant, and stir evenly to obtain a homogeneous clear oily liquid. Add the prescribed amount of medicine to the above-mentioned clear oily liquid under stirring at room temperature, stir and mix for about 1 hour to make the medicine Fully dissolve, then add supersaturated substances into it, and stir well to obtain the drug-containing liquid supersaturated self-emulsifying preparation; another precisely weighed dextran is completely dissolved in about 3000m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com