Co3O4 nano hollow sphere material and preparation method and application thereof
A hollow sphere and nanotechnology, applied in electrical components, battery electrodes, circuits, etc., can solve the problems of increased charge-discharge cycle life, irreversible capacity decay, etc. Effects of chemical actions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
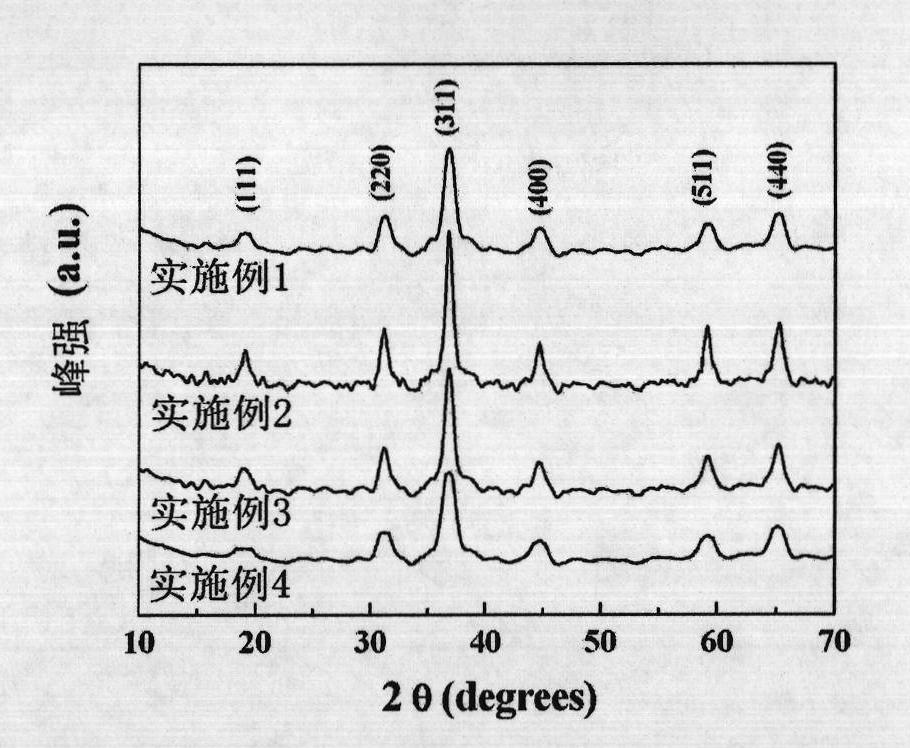
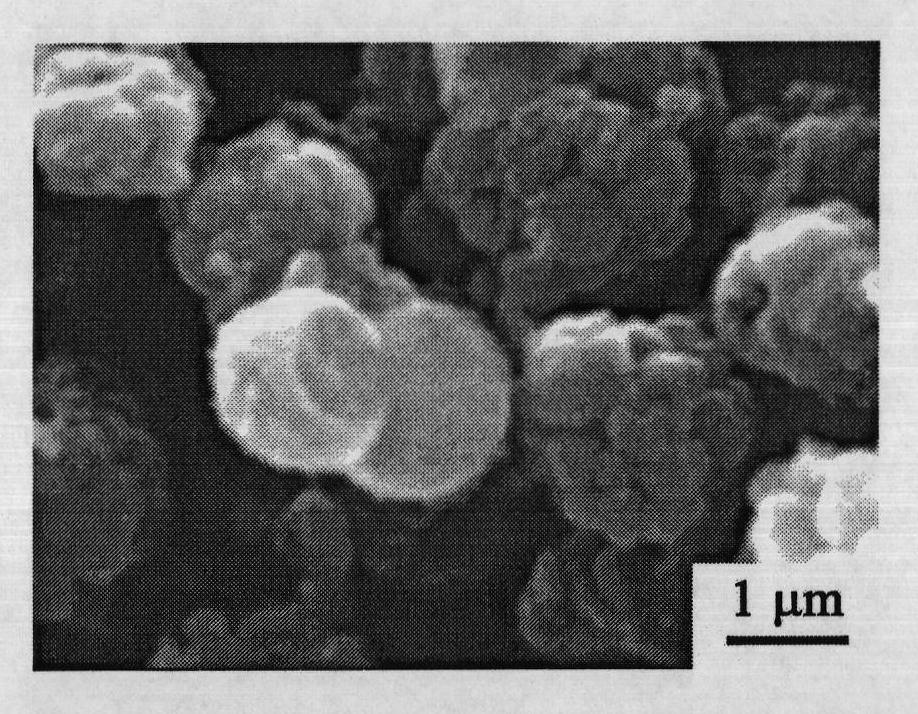
Embodiment 1
[0068] Get 5.29g cobalt nitrate (Co(NO 3 ) 2 ·6H 2 O), 1.28g hexamethylenetetramine (HMT, C 6 h 12 N 4 ), 1.79g sodium citrate (C 6 h 5 Na 3 o 7 2H 2 O) was added to 50ml deionized water, Co(NO 3 ) 2 :HMT:C 6 h 5 Na 3 o 7 The molar ratios are 6:3:2, and the entire addition process is carried out under magnetic stirring. After stirring for 60 minutes, the entire mixed solution was moved into a polytetrafluoroethylene-lined autoclave with a filling degree of 60%, reacted at a certain temperature (95° C.) for 23.5 hours, and then cooled naturally. The product was centrifuged, washed three times with deionized water and absolute ethanol, and dried at 60°C to obtain a powdery mixed precursor; the powdery mixed precursor was heat-treated at 290°C for 3 hours in an air atmosphere to obtain Final Co 3 o 4 Material.
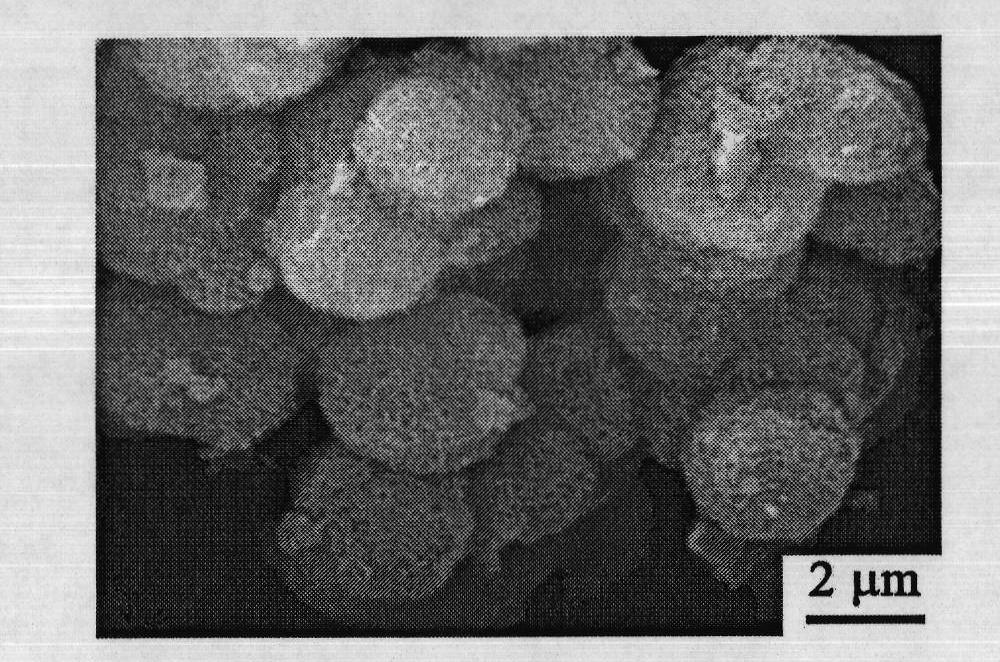
Embodiment 2
[0070] Get 5.29g cobalt nitrate (Co(NO 3 ) 2 ·6H 2 O), 2.51g hexamethylenetetramine (HMT, C 6 h 12 N 4 ), 1.79g sodium citrate (C 6 h 5 Na 3 o 7 2H 2 O) was added to 60ml deionized water, Co(NO 3 ) 2 :HMT:C 6 h 5 Na 3 o 7 The molar ratios are 3:3:1 respectively, and the whole adding process is carried out under magnetic stirring. After stirring for 30 minutes, the entire mixed solution was moved into a polytetrafluoroethylene-lined autoclave with a filling degree of 75%, reacted at a certain temperature (100° C.) for 24 hours, and then cooled naturally. The product was centrifuged, washed three times with deionized water and absolute ethanol, and dried at 70°C to obtain a powdery mixed precursor; the powdery mixed precursor was heat-treated at 300°C for 3 hours in an air atmosphere to obtain Final Co 3 o 4 Material.
Embodiment 3
[0072] Get 5.29g cobalt nitrate (Co(NO 3 ) 2 ·6H 2 O), 5.10g hexamethylenetetramine (HMT, C 6 h 12 N 4 ), 1.17g citric acid (C 6 h 8 o 7 ) into 60ml deionized water, Co(NO 3 ) 2 :HMT:C 6 h 8 o 7 The molar ratios are 3:6:1 respectively, and the whole adding process is carried out under magnetic stirring. After stirring for 45 minutes, the entire mixed solution was moved into a polytetrafluoroethylene-lined autoclave with a filling degree of 70%, reacted at a certain temperature (100° C.) for 24 hours, and then cooled naturally. The product was centrifuged, washed three times with deionized water and absolute ethanol, and dried at 80°C to obtain a powdery mixed precursor; the powdery mixed precursor was heat-treated at 300°C for 3 hours in an air atmosphere to obtain Final Co 3 o 4 Material.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com