Ionic liquid/surfactant modified glassy carbon electrode and its preparation method and application
A surfactant and ionic liquid technology, applied in the field of electroanalytical chemistry, can solve the problems of unsatisfactory preparation process and stability, and achieve the effects of avoiding modification errors, uniform film, and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Electrochemical Behavior of Bisphenol A on Ionic Liquid / Surfactant Modified Glassy Carbon Electrode
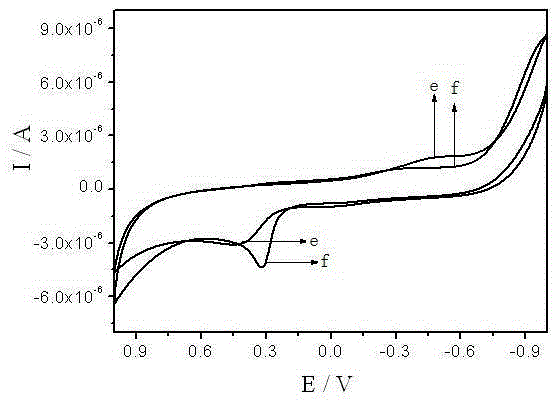
[0027] Under the conditions of controlling the potential range of -1.0-1.0V, scanning rate of 0.1V / s, and supporting electrolyte PBS buffer solution pH=8.0, the activity of bisphenol A on bare glassy carbon electrodes and modified glassy carbon electrodes was investigated by cyclic voltammetry. electrochemical behavior, figure 2 It is the cyclic voltammetry characteristic curve of bisphenol A on the ionic liquid / surfactant modified glassy carbon electrode and the bare glassy carbon electrode. It can be seen from the figure that there is only one oxidation of bisphenol A on the bare glassy carbon electrode and the modified glassy carbon electrode. peak, no reduction peak, the electrochemical response of bisphenol A on the bare glassy carbon electrode is very weak, but on the modified glassy carbon electrode, the peak type of the oxidation peak is obvious, and the peak c...
Embodiment 2
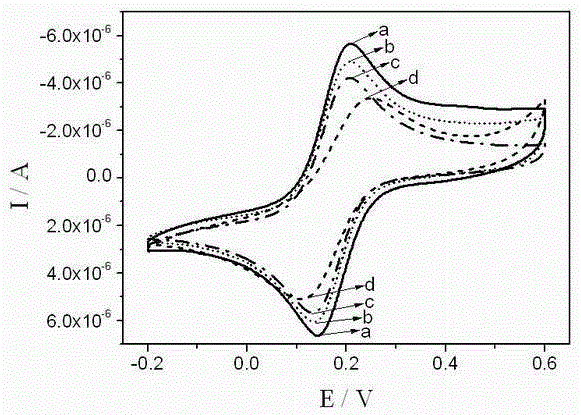
[0029] The effect of functionalized ionic liquid, surfactant, and the ratio of functionalized ionic liquid and surfactant on the electrochemical behavior of the modified electrode was explored by univariate method. The detection conditions were scan rate 0.1V / s, potential window -1.0 V-1.0V, supporting electrolyte 0.1MpH=8.0 PBS buffer solution, find out the electrode preparation conditions with the highest detection sensitivity of bisphenol A, wherein the molar concentration of surfactant cetyltrimethylammonium bromide is 1×10 -5 -5×10 -3 mol / L, functionalized ionic liquid [C n mim] BF 4 The amount used is 10-30 μL, and the volume ratio of the functionalized ionic liquid to the surfactant is 1:1-3, preferably 2:3.
Embodiment 3
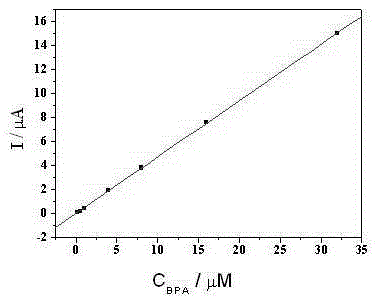
[0031] Using the modified electrode prepared by the present invention as the working electrode, explore the best experimental conditions for bisphenol A detection, including scanning speed, supporting electrolyte composition, detection potential window and enrichment time, etc., by comparing the peak current of bisphenol A oxidation under different conditions and oxidation peak potential to determine the best conditions for bisphenol A detection.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com