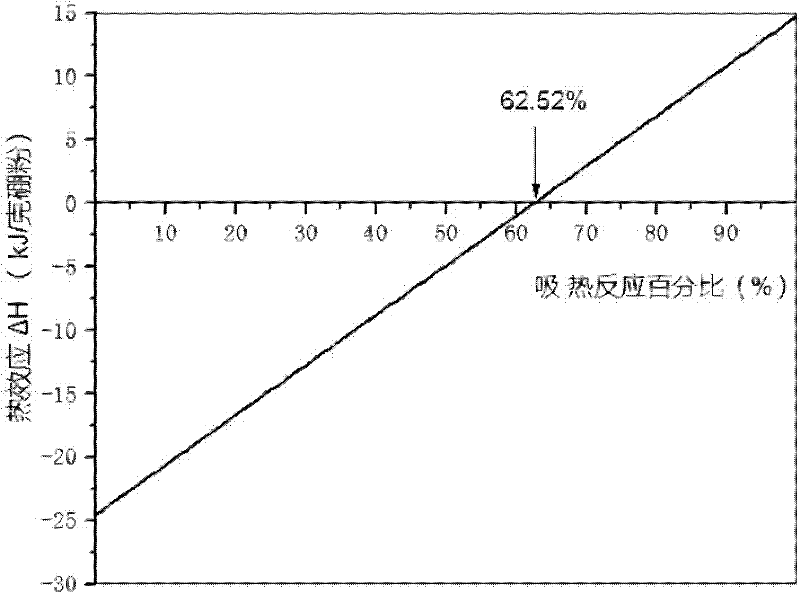
Method for preparing nano boron powder
A boron powder and B2O3 technology, applied in the field of ceramic material preparation, can solve the problems such as difficulty in improving the purity and particle size of boron powder, high consumption of raw materials for thermal explosion reaction, limited effect of regulating reaction, etc., so as to achieve particle size refinement and control. , The effect of inhibiting side reactions and by-product impurities, avoiding sintering and agglomeration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Weigh 27.38 g of B 2 o 3 , 23.64 g Mg, 7.52 g KBH 4 , the endothermic reaction rate is 30%, then the raw material mass ratio B 2 o 3 :Mg:KBH 4 1:0.86:0.28, where the B 2 o 3 It is industrial diboron trioxide powder, the size is 100-300 mesh; Mg is industrial magnesium powder, the size is 100-300 mesh; KBH 4 The purity is 96wt.%, and the size is 100-300 mesh. Add the weighed raw materials into a high-speed mixer (18000 rpm), mix for 6 minutes, and make it evenly mixed; put the mixture into a reaction tank, vibrate it and put it into an argon-protected heating furnace, and heat it at 830 Incubate at ℃ for 13 minutes to initiate a self-propagating reaction; take out the crude reaction product, add excess 0.5M hydrochloric acid to soak the crude product, and then heat and stir at 60℃ for 8 hours to dissolve impurities in the product, suction filter, wash with water several times, and filter cake Vacuum drying at 80° C. for 12 hours yielded 8.68 g of boron powder wit...
Embodiment 2
[0041] Weigh 22.57 g of B 2 o 3 , 13.52 g Mg, 15.03 g KBH 4 , the endothermic reaction rate is 60%, then the raw material mass ratio B 2 o 3 :Mg:KBH 4 1:0.60:0.67, where the B 2 o 3 It is industrial diboron trioxide powder, the size is 100-300 mesh; Mg is industrial magnesium powder, the size is 100-300 mesh; KBH 4 The purity is 97wt.%, and the size is 100-300 mesh. Add the weighed raw materials into a high-speed mixer (18,000 rpm), mix for 10 minutes, and make it evenly mixed; put the mixture into a reaction tank, vibrate it and place it in an argon-protected heating furnace, and heat it at 800 Incubate at ℃ for 15 minutes to initiate a self-propagating reaction; take out the crude reaction product, add 0.5M hydrochloric acid to soak the crude product, and then heat and stir at 60℃ for 12 hours to dissolve impurities in the product, suction filter and wash with water several times, and filter cake at 80 °C for 16 hours in vacuum to obtain 8.89 g of boron powder with a...
Embodiment 3
[0043] Weigh 25.80 g of B 2 o 3 , 20.27 g Mg, 10.05 g KBH 4 , the endothermic reaction rate is 40%, then the raw material mass ratio B 2 o 3 :Mg:KBH 4 1:0.79:0.39, where the B 2 o 3 It is industrial diboron trioxide powder, the size is 100-300 mesh; Mg is industrial magnesium powder, the size is 100-300 mesh; KBH 4 The purity is 96wt.%, and the size is 100-300 mesh. Add the weighed raw materials into a high-speed mixer (18000 rpm), mix for 12 minutes, and make it evenly mixed; put the mixture into a reaction tank, vibrate it and put it into an argon-protected heating furnace, and heat it at 750 Incubate at ℃ for 15 minutes to initiate a self-propagating reaction; take out the crude reaction product, add 5M hydrochloric acid to soak the crude reaction product, and then heat and stir at 60 ℃ for 16 hours to dissolve impurities in the product, filter with suction, wash with water several times, and filter the cake at 80 ℃ Vacuum dried for 24 hours to obtain 8.67 g of boro...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| heating value | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com