Immunomodifier for reducing blood total cholesterol and administration method thereof
A technology of total cholesterol and cholesterol, applied in the direction of drug combination, metabolic diseases, bacterial antigen components, etc., can solve the problem that there is no specific difference between immune pathways and immune strategies
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
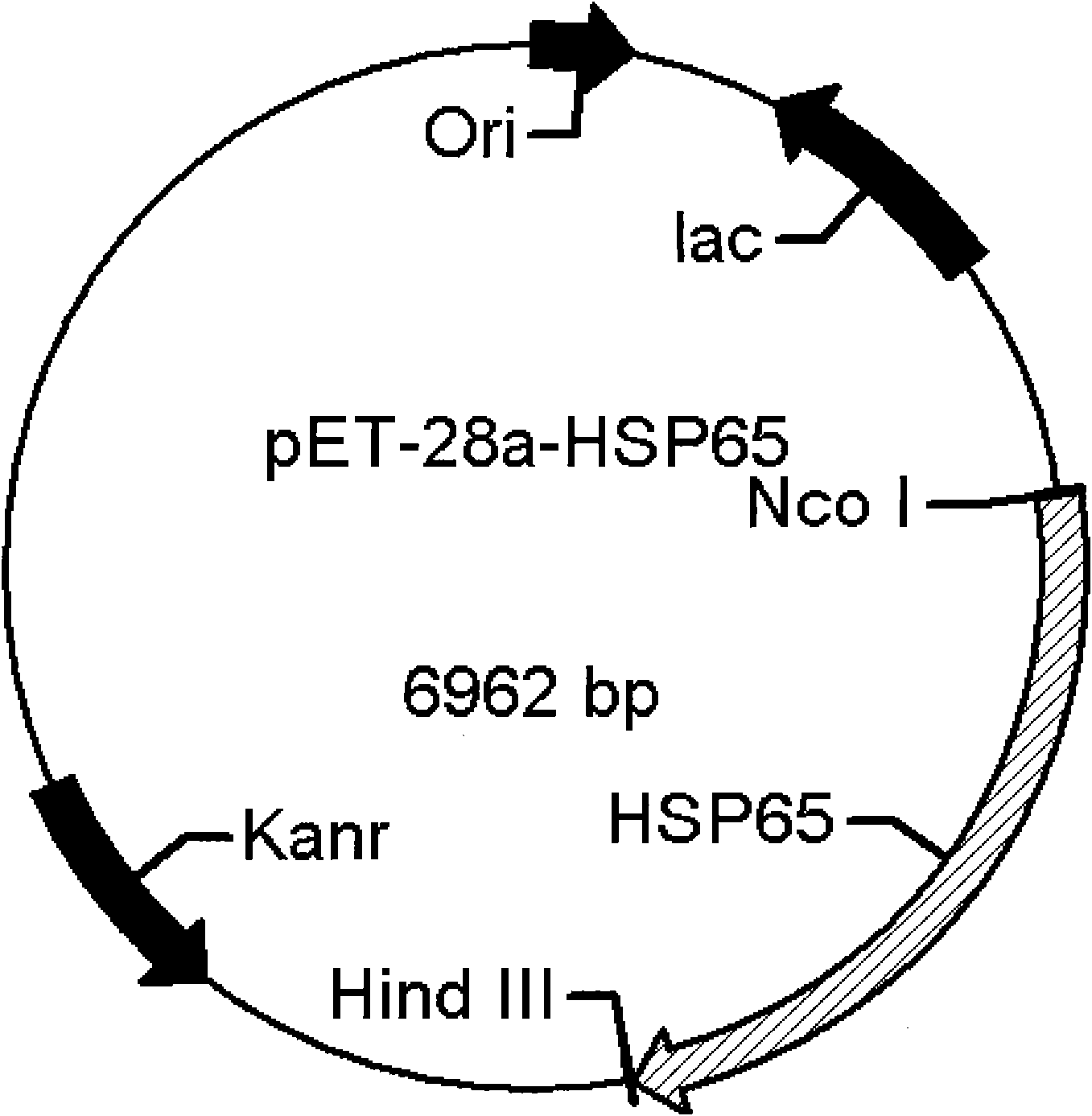
[0042] Embodiment 1: Cloning of HSP65 gene
[0043] Genomic DNA was extracted from commercially available BCG (refer to the "Refined Molecular Biology Experiment Guide" for specific steps), and the full gene sequence of HSP65 in BCG was found by National Center for Biotechnology Information (NCBI). Under the premise of not changing the amino acid sequence of HSP65, codons favored by Escherichia coli were selected, and two primers P1 and P2 were designed with the help of computer software.
[0044] The nucleotide sequences of the two primers are as follows:
[0045] P1: 5'TTCG CCATGG CCAAHACA ATTGCGTACG 3’
[0046] P2: 5'TTCCG AAGCTT AGAAATCCATGCCACCCATG 3'
[0047] An NcoI restriction site was introduced into the upstream primer P1, and a HindIII restriction site was introduced into the downstream primer P2.
[0048] The PCR reaction system is: 100 pmol P1, 100 pmol P2, 2 μg BCG genomic DNA, 2 μl dNTP (10 mM), 5 μl 10×Pfu buffer and 5 units of Pfu DNA polymerase, the to...
Embodiment 2
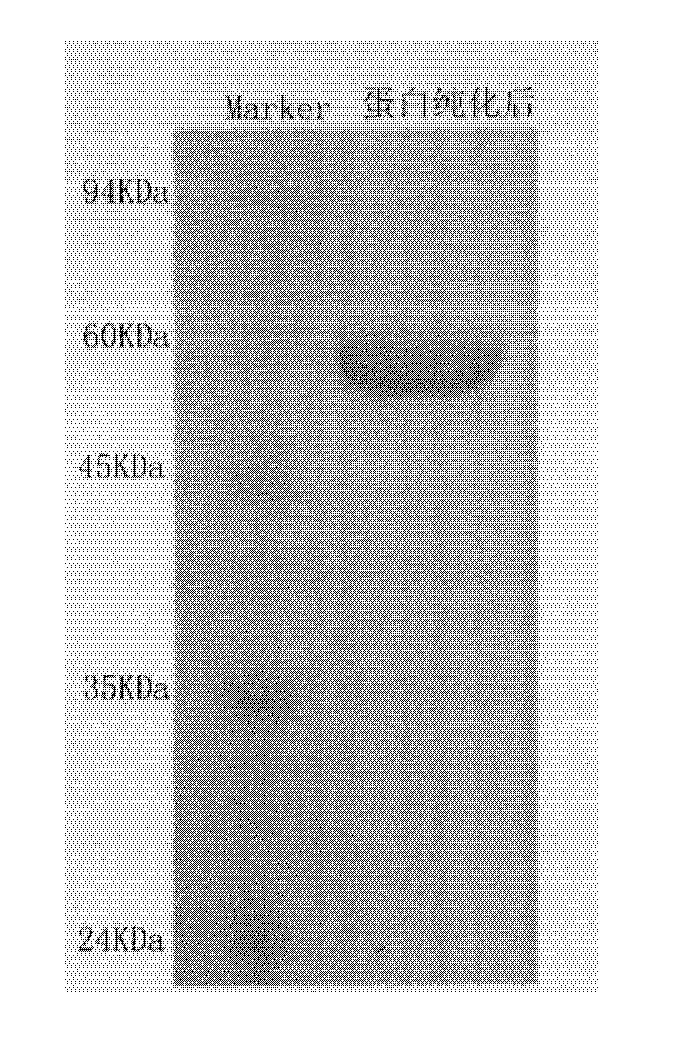
[0050] Example 2: Expression of HSP65 in Escherichia coli
[0051] Pick a single colony from the pET-28a-HSP65 plate and inoculate it into 5ml of LB medium containing 50 μg / ml kanamycin, cultivate overnight at 37°C with constant temperature shaking, and transfer to fresh corn steep liquor liquid medium (50 μg / ml kanamycin), after culturing at 37°C for 4 hours, α-lactose with a final concentration of 5mmol / L was added to induce recombinant E. coli to express protein HSP65. After 6 hours of induction, the engineered bacteria recovered the thalline through centrifugation, suspended the thalline in the thalline lysate (pH8.0, 10mmol / L Tris-HCl buffer solution, 0.02% lysozyme), freeze-thawed once, added DNase I to The DNA was digested until the solution was no longer viscous, the supernatant was recovered by centrifugation, the ammonium sulfate was fractionated and precipitated, and 35-40% of the precipitate was redissolved in the ion exchange buffer (10mmol / L Tris-HCl, pH8.0), an...
Embodiment 4
[0052] Embodiment 4: Pharmacodynamic study of HSP65 recombinant protein
[0053] Twenty-four 6-week-old male C57BL / 6 mice were randomly divided into 3 groups, 8 mice in each group, which were PBS blank group, OVA (ovalbumin) nasal mucosa immunization group and HSP65 nasal mucosa immunization group. Animals were fed with high-fat and high-cholesterol feed (10% lard, 2% cholesterol and 0.5% bile salt were added to common mouse feed). Immunization started after 20 days, once a day for 10 consecutive days. The HSP65 recombinant protein was dissolved in sterilized PBS, quantified by the Coomassie Brilliant Blue Kit, configured to 1 μg / μl, and instilled from the nasal cavity, 20 μl per mouse, containing 20 μg protein. The change of serum cholesterol after immunization was detected. Blood was collected from the retro-orbital venous plexus 14 days after the 10th immunization, about 0.2ml per mouse, centrifuged at 4000 rpm for 10 minutes, and the serum was collected. The total chole...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com