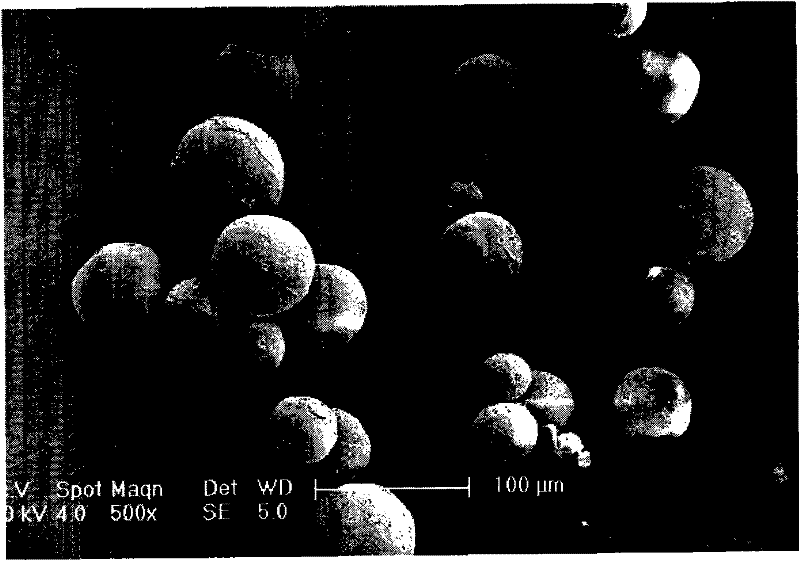
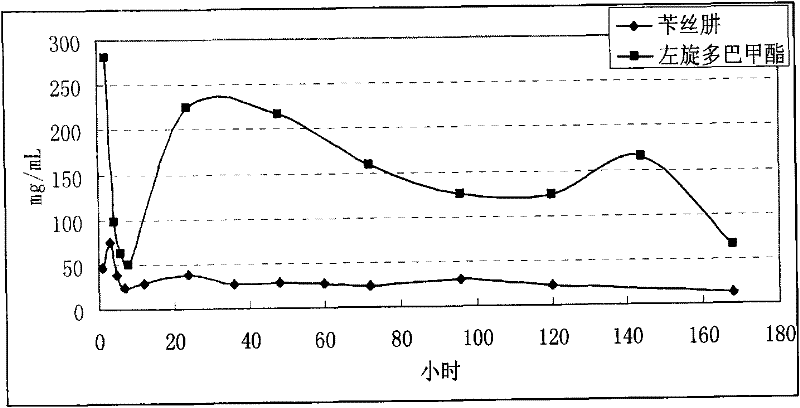
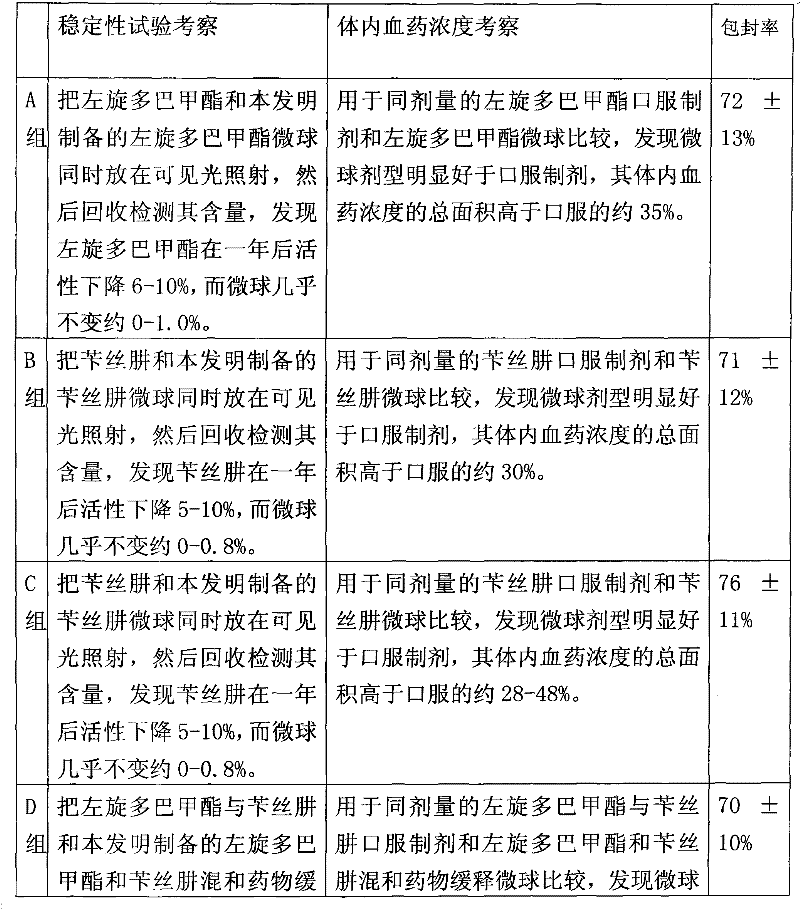
Levodopa methyl ester and benserazide mixed medicament slow-release microsphere composition and preparation method thereof
A technology of levodopa methyl ester and slow-release microspheres, which is applied in the direction of drug combinations, pharmaceutical formulas, and medical preparations containing active ingredients, etc., can solve the problems of inactivation and non-preparation of levodopa methyl ester, and achieve re- good dispersion effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] ①Preparation of mixed drug solution of levodopa methyl ester and benserazide
[0029] a) Mix 100 mg of levodopa methyl ester and benserazide (according to the weight ratio of 1:1) purchased in the market, and prepare the concentration of 2.5% by weight;
[0030] ②Preparation of sustained-release microsphere composition of levodopa methyl ester and benserazide mixed drug
[0031] a) Measure 0.2mL, 0.5mL or 1mL of the mixed drug solution of levodopa methyl ester and benserazide obtained in ①, and weigh 595mg of polyglycolic acid-polylactic acid (PLGA molecular weight is 6000), 37.5mg of Polyglycolic acid-polylactic acid (PLGA molecular weight is 250,000) or 25 mg of polyglycolic acid-polylactic acid (PLGA molecular weight is 500,000) and is prepared into an organic solution of 30%, 15% or 5% by weight concentration of dichloromethane respectively 30% concentration and 0.2mL, 15% and 0.5mL or 5% and 1mL of the above solutions are mixed one by one correspondingly and stirr...
Embodiment 2
[0039] ①Preparation of mixed drug solution of levodopa methyl ester and benserazide
[0040]a) Mixing 100 mg of levodopa methyl ester and benserazide (according to the weight ratio of 2: 1) purchased on the market to prepare a concentration of 2.5% by weight;
[0041] ②Preparation of sustained-release microsphere composition of levodopa methyl ester and benserazide mixed drug
[0042] a) Measure 0.2mL, 0.5mL or 1mL of the mixed drug solution of levodopa methyl ester and benserazide obtained in ① respectively and weigh 595mg of polylactic acid (PLA molecular weight is 6000), 37.5mg of polylactic acid (PLA Molecular weight is 250,000) or polylactic acid (PLA molecular weight is 500,000) of 25mg and is prepared into the organic solution of the methylene chloride of 30%, 15% or 5% by weight percentage concentration respectively; Will use 30% concentration and 0.2mL, 15 % and 0.5mL or 5% and 1mL of the above solutions are mixed one by one and stirred, vortexed or ultrasonically fo...
Embodiment 3
[0050] ①Preparation of mixed drug solution of levodopa methyl ester and benserazide
[0051] a) Mix 100 mg of levodopa methyl ester and benserazide (according to the weight ratio of 3:1) purchased in the market, and prepare the concentration of 2.5% by weight;
[0052] ②Preparation of sustained-release microsphere composition of levodopa methyl ester and benserazide mixed drug
[0053] a) Measure 0.2mL, 0.5mL or 1mL of the mixed drug solution of levodopa methyl ester and benserazide obtained in ①, and weigh 595mg of polycaprolactone (PCL molecular weight is 10,000), 37.5mg of polycaprolactone Lactone (PCL molecular weight is 2,500,000) or 25mg of polycaprolactone (PCL molecular weight is 5,000,000) and is prepared into an organic solution of dichloromethane with a concentration of 30%, 15% or 5% by weight; 30% concentration will be used and 0.2mL, 15% and 0.5mL or 5% and 1mL of the above solutions in the order of one-to-one mixing and stirring, vortex or ultrasonic 1-5 minute...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com