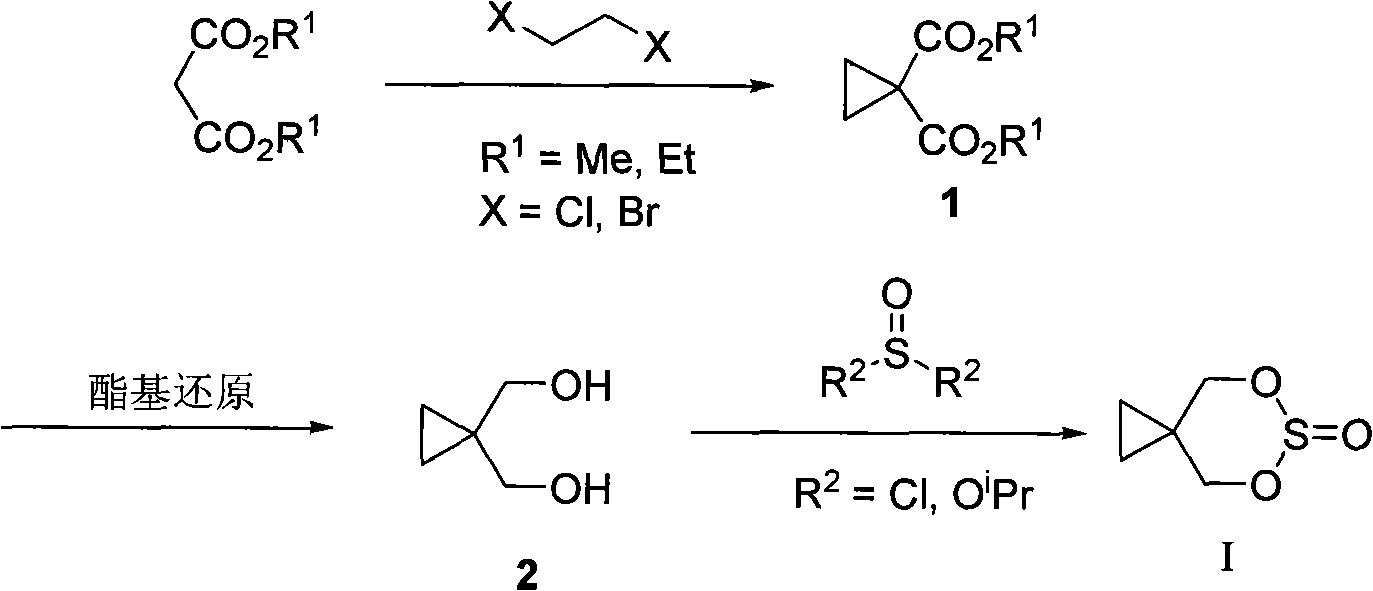
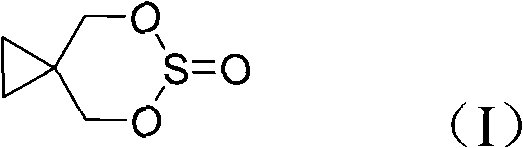Method for preparing 1,1-cyclopropanedimethyl cyclicsulfite
A technology for methanol cyclic sulfite and body cyclic sulfite, applied in the field of preparation 1, can solve the problems of complicated processing, high cost, many reaction steps, etc., and achieves high reaction yield, low cost, and is conducive to industrial production. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] A method for preparing 1,1-cyclopropane dimethanol cyclosulfite, the specific steps are:
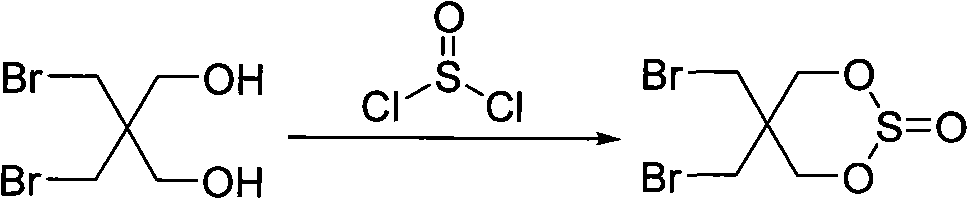
[0043] Step 1, synthetic intermediate cyclic sulfite, its general reaction chemical formula is as follows:
[0044]
[0045] Specifically: after mixing dibromoneopentyl glycol (200 g, 0.76 mol) with 350 ml of toluene, heat to 40° C.;
[0046] Thionyl chloride (100 grams, 0.84 moles) was added dropwise, and the temperature of the solution rose to above 45°C due to the mild exothermic reaction. °C, drop the thionyl chloride in about 1 hour; during the reaction, use lye to absorb the hydrogen chloride (HCl) gas generated after the solid gradually dissolves;
[0047] Then the reaction mixture was heated to 50°C for 2.5 hours;
[0048] After completion of the reaction, the toluene solution was evaporated under reduced pressure, and the resulting oily liquid solidified after cooling and standing; after the solid was pulverized, it was stirred and washed with water (1 liter × 3) (i....
Embodiment 2
[0055] A method for preparing 1,1-cyclopropane dimethanol cyclosulfite, the specific steps are:
[0056] Step 1, synthetic intermediate cyclic sulfite, its general reaction chemical formula is as follows:
[0057]
[0058] Specifically: after mixing dibromoneopentyl glycol (200 g, 0.76 mol) with 350 ml of toluene, heat to 40° C.;
[0059] Thionyl chloride (100 grams, 0.84 moles) was added dropwise, and the temperature of the solution rose to above 45°C due to the mild exothermic reaction. °C, drop the thionyl chloride in about 1 hour; during the reaction, use lye to absorb the hydrogen chloride (HCl) gas generated after the solid gradually dissolves;
[0060] Then the reaction mixture was heated to 50°C for 2.5 hours;
[0061] After the reaction was completed, the toluene solution was evaporated to dryness under reduced pressure, and the obtained oily liquid solidified after cooling and standing; after the obtained solid was pulverized, washed with water (1 liter × 3), fi...
Embodiment 3
[0068] A method for preparing 1,1-cyclopropane dimethanol cyclosulfite, the specific steps are:
[0069] Step 1, synthetic intermediate cyclic sulfite, its general reaction chemical formula is as follows:
[0070]
[0071] Specifically: after mixing dibromoneopentyl glycol (200 g, 0.76 mol) with 350 ml of toluene, heat to 40° C.;
[0072] Thionyl chloride (100 grams, 0.84 moles) was added dropwise, and the temperature of the solution rose to above 45°C due to the mild exothermic reaction. °C, drop the thionyl chloride in about 1 hour; during the reaction, use lye to absorb the hydrogen chloride (HCl) gas generated after the solid gradually dissolves;
[0073] Then the reaction mixture was heated to 50°C for 2.5 hours;
[0074] After the reaction was completed, the toluene solution was evaporated to dryness under reduced pressure, and the obtained oily liquid solidified after cooling and standing; after the obtained solid was pulverized, washed with water (1 liter × 3), fi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com