Method for continuously enriching and separating phosphoeptide at high throughput
A phosphopeptide, high-throughput technology, applied in peptide preparation methods, chemical instruments and methods, peptides, etc., can solve difficult phosphopeptide continuous, high-throughput separation and enrichment, time-consuming and laborious, difficult to achieve selectivity and other problems, to achieve high-throughput separation and enrichment, to avoid manual operation, and to achieve the effect of simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
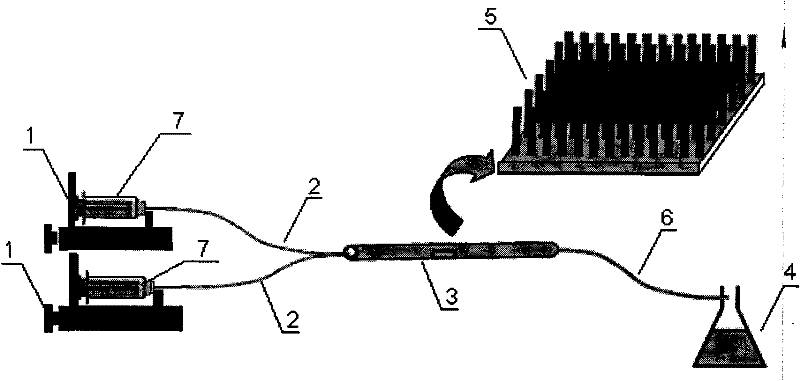
[0042] Use a ruler to measure a quartz capillary with a length of 8 cm, an inner diameter of 530 μm, and an outer diameter of 660 μm. Put 0.05M zinc acetate and 0.05M hexamethylenetetramine solutions into two syringes placed on the micro-injection pump respectively, set the pushing speed at 25 μL / min, and transport the two solutions to the oven at 90 °C at the same time. In the capillary microchannel in , the fluid delivery time was controlled to be 2h, and vertically grown ZnO nanorods were prepared on the inner surface of the microchannel. Then, 65 μg / mL of TiO was delivered into the microchannel at a syringe pump speed of 5 μL / min and an oven temperature of 80 °C. 2 Sol, control the fluid delivery time for 12h, and obtain vertically grown TiO after drying 2 / ZnO nanorod arrays. figure 1 The TiO grown on the inner surface of the microchannel obtained for this embodiment 2 / ZnO nanorod array field emission scanning electron microscope photograph, it can be seen that the n...
Embodiment 2
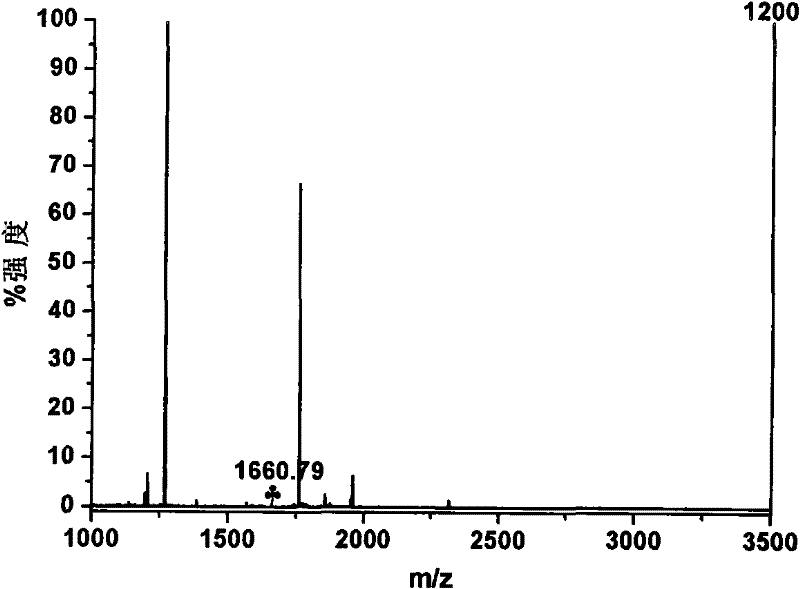
[0046] Adjust enrichment residence time to be 30s and continue 3min, other conditions are the same as embodiment 1. Figure 5 is the MALDI-TOF MS spectrum after enrichment of 500fmolα-casein hydrolyzate in this example, wherein The number is the characteristic peak of phosphopeptide. It is not difficult to find that when the enrichment residence time is 30s, the signals of the three characteristic peaks of α-casein phosphopeptides in the spectrum are enhanced, and the signals of non-phosphorylated peptides are reduced, indicating that the microfluidic device can detect phosphopeptides Perform fast and efficient enrichment.
Embodiment 3
[0048] Use a ruler to measure a quartz capillary with a length of 12 cm, an inner diameter of 530 μm, and an outer diameter of 660 μm. Put the 0.05M zinc acetate and 0.05M hexamethylenetetramine solutions into two syringes placed on the micro-injection pump respectively, set the pushing speed at 10 μL / min, and transfer the two solutions to the oven at 90 °C at the same time. In the capillary microchannel in , the fluid delivery time was controlled to be 5h, and vertically grown ZnO nanorods were prepared on the inner surface of the microchannel. Then, 65 μg / mL of TiO was delivered into the microchannel at a syringe pump speed of 5 μL / min and an oven temperature of 70 °C. 2 Sol, control the fluid delivery time for 12h, and obtain vertically grown TiO after drying 2 / ZnO nanorod arrays.
[0049] Take two polytetrafluoroethylene microtubes with an inner diameter of 300 μm and a length of 40 cm as fluid delivery channels, and glue them to one end of the quartz capillary with epo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com