Synthesizing method of N-beta-alanyl-(tau-methyl) histidine
A synthetic method and technology of histidine, applied in the direction of organic chemistry, etc., can solve problems such as methylation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
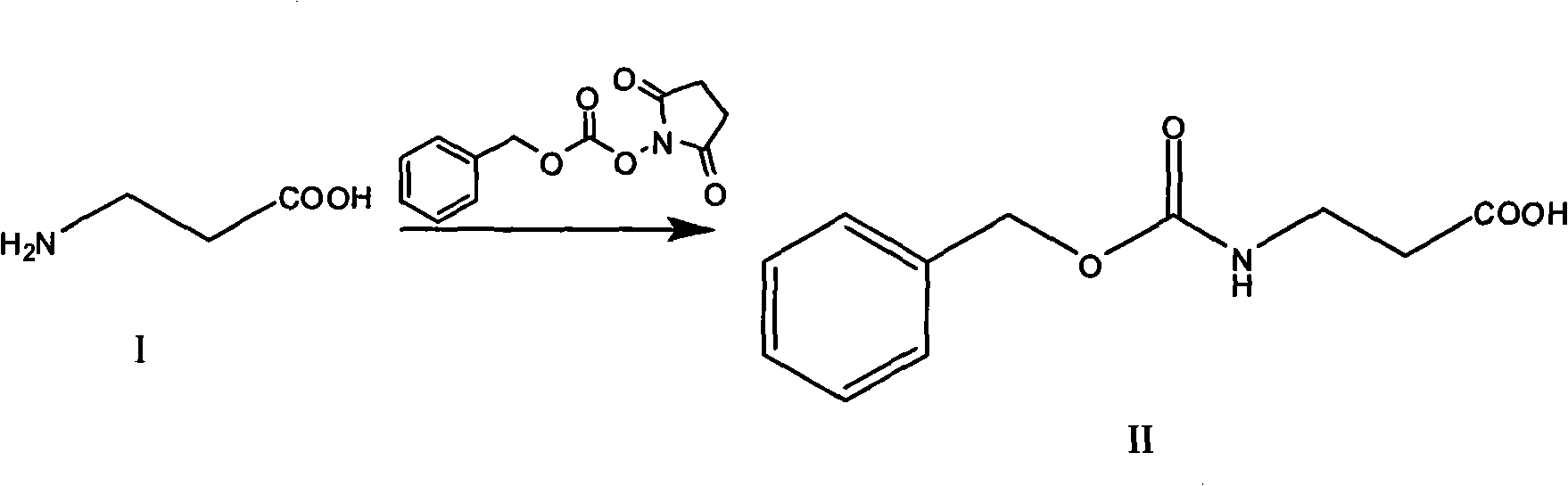
[0021] (1) Preparation of N-benzyloxycarbonyl-β-alanine
[0022] Add 750ml of water, 150 grams (1.685mol) of β-alanine, 212 grams (2.527mol) of sodium bicarbonate and 440 grams of benzyloxycarbonyl succinimide in a beaker, and use 2mol / L sodium hydroxide solution Control the pH value between 8 and 9, extract impurities with ether after the reaction is complete, and discard the ether phase. The aqueous phase was extracted with ethyl acetate, washed with saturated brine, dried over anhydrous sodium sulfate, evaporated to remove most of the ethyl acetate, frozen overnight, filtered to obtain 208 g of white crystals, yield: 55.3%.
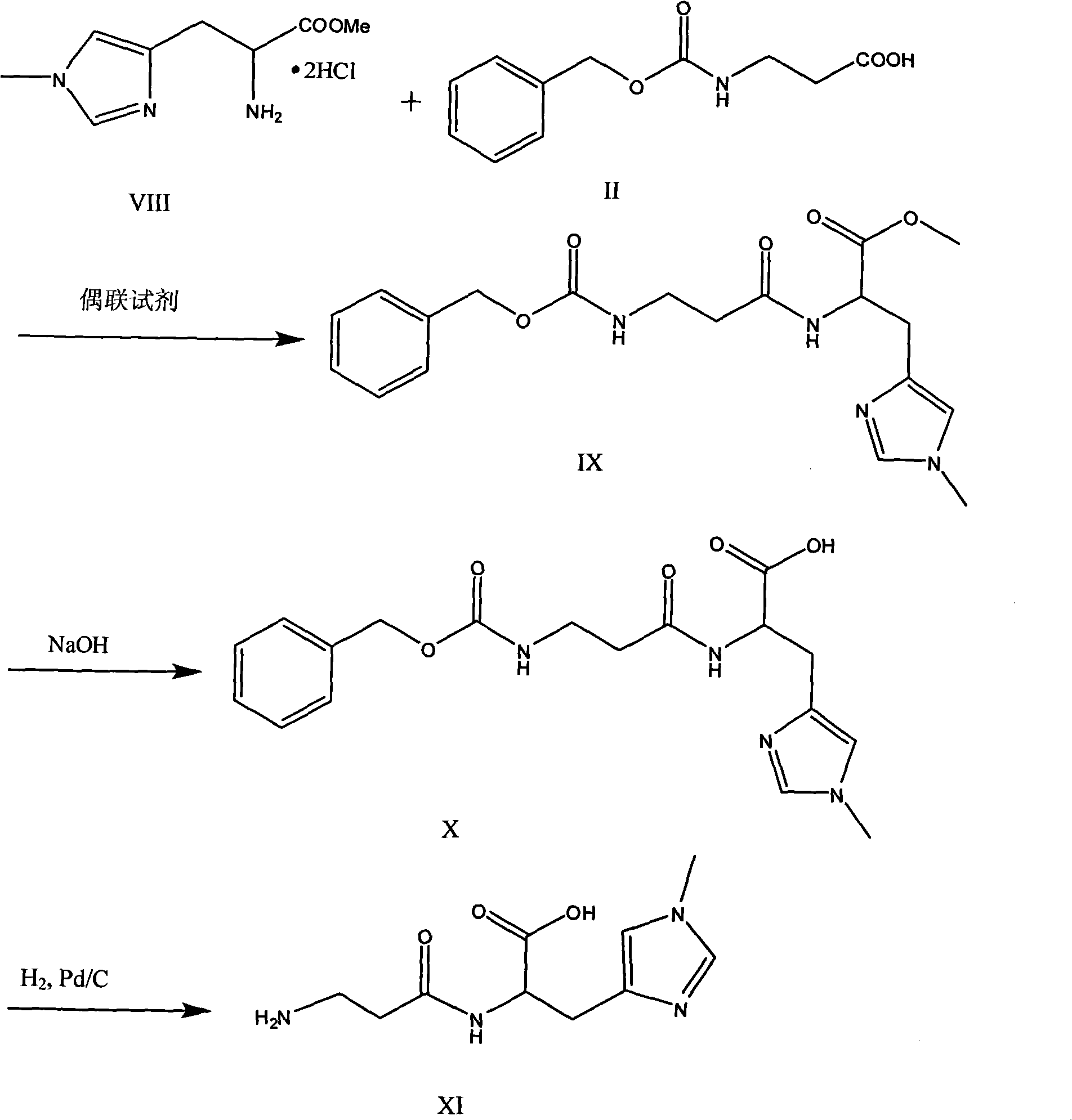
[0023] (2) Preparation of histidine methyl ester hydrochloride
[0024] Add 6.5L of anhydrous methanol to a 10L three-necked flask, add 620 grams (4mol) of L-histidine, and dropwise add 855ml (12mol) of thionyl chloride. After the reaction is completed, directly transfer it to a 20L clean container, add 5L ether, filter 940 g of white crystals were o...
Embodiment 2
[0040] (1) Preparation of N-benzyloxycarbonyl-β-alanine
[0041] Add 750ml of water, 150 grams (1.685mol) of β-alanine, 212 grams (2.527mol) of sodium bicarbonate and 440 grams of benzyloxycarbonyl succinimide in a beaker, and use 2mol / L sodium hydroxide solution Control the pH value between 8 and 9, extract impurities with ether after the reaction is complete, and discard the ether phase. The aqueous phase was extracted with ethyl acetate, washed with saturated brine, dried over anhydrous sodium sulfate, evaporated to remove most of the ethyl acetate, frozen overnight, filtered to obtain 208 g of white crystals, yield: 55.3%.
[0042] (2) Preparation of histidine methyl ester hydrochloride
[0043] Add 6.5L of anhydrous methanol to a 10L three-necked flask, add 620 grams (4mol) of L-histidine, and dropwise add 855ml (12mol) of thionyl chloride. After the reaction is completed, directly transfer it to a 20L clean container, add 5L ether, filter 940 g of white crystals were o...
Embodiment 3
[0059] (1) Preparation of N-benzyloxycarbonyl-β-alanine
[0060] Add 750ml of water, 150 grams (1.685mol) of β-alanine, 212 grams (2.527mol) of sodium bicarbonate and 440 grams of benzyloxycarbonyl succinimide in a beaker, and use 2mol / L sodium hydroxide solution Control the pH value between 8 and 9, extract impurities with ether after the reaction is complete, and discard the ether phase. The aqueous phase was extracted with ethyl acetate, washed with saturated brine, dried over anhydrous sodium sulfate, evaporated to remove most of the ethyl acetate, frozen overnight, filtered to obtain 208 g of white crystals, yield: 55.3%.
[0061] (2) Preparation of histidine methyl ester hydrochloride
[0062] Add 6.5L of anhydrous methanol to a 10L three-necked flask, add 620 grams (4mol) of L-histidine, and dropwise add 855ml (12mol) of thionyl chloride. After the reaction is completed, directly transfer it to a 20L clean container, add 5L ether, filter 940 g of white crystals were o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com