Catalyst for combustion of ventilation air methane and preparation method thereof
A technology for burning catalysts and wind methane, applied in chemical instruments and methods, physical/chemical process catalysts, separation methods, etc., can solve the problems of catalyst catalytic activity limitation, complex process, complex preparation methods, etc., to meet thermal stability and Requirements for low-temperature activity, cost reduction, and the effect of simple preparation methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
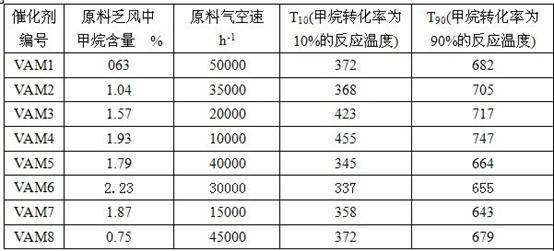
Examples
Embodiment 1
[0039] The composition of present embodiment catalyst is:
[0040] 1% active component, 30% coagent and 69% carrier, the active component is ferric nitrate equivalent to 1% ferric oxide, and the coagent is cerium nitrate equivalent to 30% cerium oxide , the carrier is 69% alumina, named VAM1.
[0041] The catalyst is prepared by the following method:
[0042] (1), 5.1g ferric nitrate nonahydrate (equivalent to 1g ferric oxide), 76g cerium nitrate hexahydrate (equivalent to 30g cerium dioxide) were dissolved in 100g concentration of 5wt% citric acid aqueous solution, made into solution;
[0043] (2), immerse 69g of alumina in the aqueous solution prepared in step (1), and immerse at room temperature for 4 hours;
[0044] (3) Extrude the mixture in step (2), dry at 120°C for 1 hour, and calcinate at 500°C for 4 hours to obtain a strip catalyst.
Embodiment 2
[0046] The composition of present embodiment catalyst is:
[0047] 50% active component, 1% coagent and 49% carrier, the active component is ferrous sulfate equivalent to 50% of the content of ferric oxide in the catalyst, and the coagent is equivalent to the content of dilanthanum trioxide It is 1% lanthanum nitrate, the carrier is composed of 40% titanium oxide and 9% zirconium oxide, named VAM2.
[0048] The catalyst is prepared by the following method:
[0049] (1), 2.7 g of lanthanum nitrate hexahydrate (equivalent to 1 g of lanthanum trioxide) was dissolved in 50 g of citric acid aqueous solution with a concentration of 1 wt%, to form a solution;
[0050] (2) Dissolve 40g of titanium oxide and 9g of zirconium oxide in the aqueous solution prepared in step (1), immerse at room temperature for 3 hours, dry naturally, press into tablets, dry at 120°C for 2 hours, and bake at 500°C for 5 hours , that is, the catalyst precursor is obtained.
[0051] (3) Dissolve 174.2g of ...
Embodiment 3
[0053] The composition of present embodiment catalyst is:
[0054] 40% active component, 20% coagent and 40% carrier, the active component is 40% copper oxide, the coagent is composed of lanthanum nitrate equivalent to 20% lanthanum trioxide, and the carrier is 20% Composed of magnesium oxide and 20% calcium oxide, named VAM3.
[0055] The catalyst is prepared by the following method:
[0056] (1) Fully mix 40g of copper oxide, 20g of magnesium oxide and 20g of calcium oxide.
[0057] (2) The mixture is rolled into a ball, dried at 120°C for 2 hours, and calcined at 500°C for 2 hours to make a catalyst precursor;
[0058] (3) Dissolve 54g of lanthanum nitrate hexahydrate (equivalent to 20g of dilanthanum trioxide) in 150g of citric acid aqueous solution with a concentration of 10wt% to form a solution. The catalyst precursor in step (2) was immersed in the solution, soaked at room temperature for 6 hours, dried at 120°C for 2 hours, and calcined at 700°C for 4 hours to obta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com