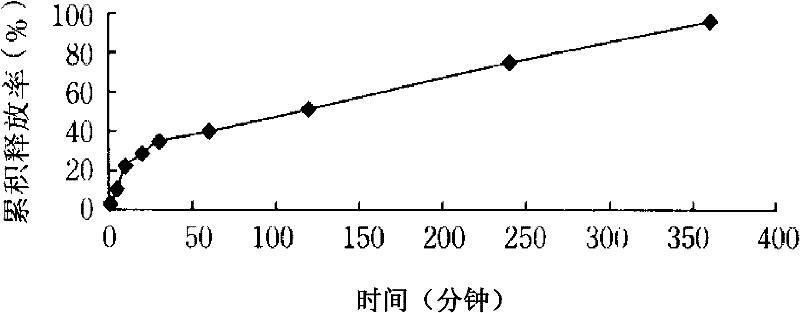
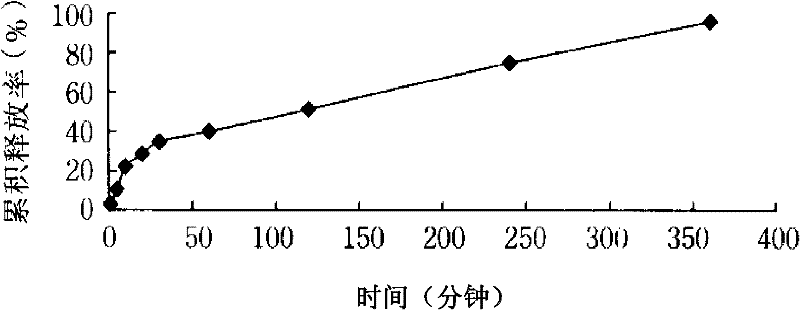
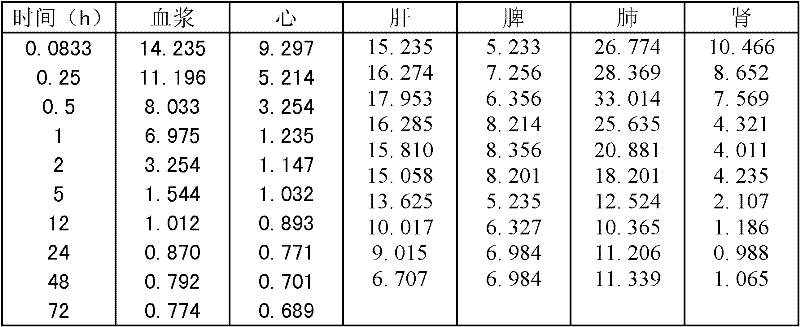
Method for preparing tetrandrine lung-targeted controlled-release microspheres
A tetrandrine, lung-targeting technology, applied in the field of medicine and health, can solve the problems of low drug loading, failure to obtain pharmacodynamic effect and controlled release effect at the same time, and large particle size of microspheres, and achieve drug loading rate. The effect of high and low blood drug concentration, distribution in non-affected tissue, and stable quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] (1) Tetrandrine is used as the drug and polylactic acid is used as the polymer excipient, and the drug and the polymer excipient are dissolved respectively to form a drug solution and a polymer excipient solution; wherein the concentration of the polylactic acid solution is 5%.
[0025] (2) Mix the drug solution and the polymer auxiliary material solution according to the volume ratio of 8:100, and stir evenly; the weight ratio of tetrandrine and polylactic acid in the mixed solution is 5:7.
[0026] (3) Ultrasonic emulsification: put the mixed liquid in a glass container, turn on the ultrasonic generator, the power is 5 kilowatts, and the emulsification time is 20 minutes.
[0027] (4) Coagulation: the liquid is stirred at a stirring speed of 600 rpm for 1 hour.
[0028] (5) Solidification: Add glutaraldehyde to the liquid to solidify the microspheres, and stir continuously at a stirring speed of 500 rpm for 1 hour.
[0029] (6) Sedimentation: The prepared microsphere...
Embodiment 2
[0033] (1) Tetrandrine is used as a drug and polylactic acid is used as a polymer excipient, and the drug and the polymer excipient are dissolved respectively to form a drug solution and a polymer excipient solution; wherein the concentration of the polylactic acid solution is 2%.
[0034] (2) Mix the drug solution and the polymer auxiliary material solution according to the volume ratio of 8:100, and stir evenly; the weight ratio of tetrandrine and polylactic acid in the mixed solution is 5:8.
[0035] (3) Ultrasonic emulsification: put the mixed liquid in a glass container, turn on the ultrasonic generator, the power is 5 kW, and the emulsification time is 10 minutes.
[0036] (4) Coagulation: the liquid is stirred at a stirring speed of 600 rpm for 1 hour.
[0037] (5) Solidification: Add glutaraldehyde to the liquid to solidify the microspheres, and stir continuously at a stirring speed of 500 rpm for 1 hour.
[0038] (6) Sedimentation: The prepared microspheres are settl...
Embodiment 3
[0042] (1) Tetrandrine is used as a drug, and chitosan is used as a polymer auxiliary material, and the drug and the polymer auxiliary material are dissolved respectively to form a drug solution and a polymer auxiliary material solution; wherein the concentration of the chitosan solution is 3.5%.
[0043] (2) Mix the drug solution and the polymer auxiliary material solution according to a volume ratio of 12:100, and stir evenly; the weight ratio of tetrandrine and polylactic acid in the mixed solution is 5:9.
[0044] (3) Ultrasonic emulsification: put the mixed liquid in a glass container, turn on the ultrasonic generator, the power is 5 kilowatts, and the emulsification time is 20 minutes.
[0045] (4) Coagulation: the liquid is stirred at a stirring speed of 600 rpm for 1 hour.
[0046] (5) Solidification: Add glutaraldehyde to the liquid to solidify the microspheres, and stir continuously at a stirring speed of 500 rpm for 1 hour.
[0047] (6) Sedimentation: The prepared ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| encapsulation rate | aaaaa | aaaaa |
| encapsulation rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com